Breaking down the microbiology world one bite at a time
Recurrent UTIs and GI microbiome
Relieving oneself is one of the simple pleasures of life. It’s a time to relax, be vulnerable, and to be human. Unfortunately, 50-60% of women and 12% of men will, at least once in their lifetime, have this experience ruined by the burning sensation caused by a urinary tract infection (UTI). What’s worse, is that within 20-30% of women who experience a UTI, they can experience recurrent infections, up to six times a year!
But we live in the age of antibiotics, it’s an easy fix, right? Well, no. Recurrent UTIs (rUTI) become increasingly difficult to treat as more resilient bacteria evade treatment. Worse, the likelihood of developing a UTI begins to increase with age (especially in post-menopausal women). This leads to a reduced quality of life thanks to the psychological and sociological effects.
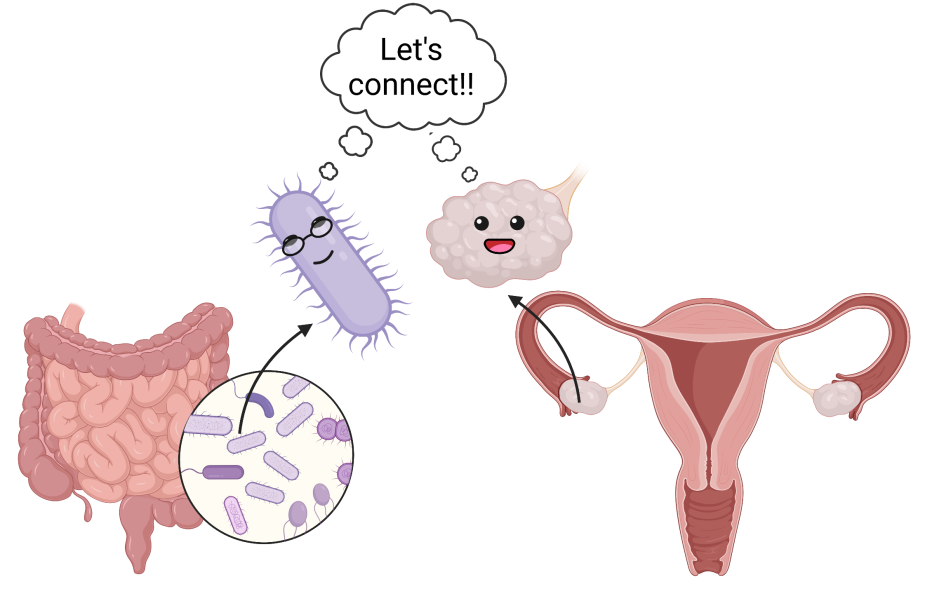
What makes some women more susceptible to recurrent infections? A variety of factors have been identified and it’s a good idea to check out what preventative measures can be taken. In the past decade, studies have suggested that the shifts in the gut microbiome, which has been linked with disturbances in other organ systems, could also play a role in rUTI.

The set up
To explore this relationship Worby et al., created the UTI microbiome project (UMB), a Cohort Study, also known as a Prospective Observational Study. The clinical trial was conducted in two groups, one experimental and another control. The experimental cohort, composed of 15 women, is classified as having a minimum of three UTIs in the past year. The control cohort comprised of 16 women of similar age and health status. Upon enrollment stool, blood, and urine samples were collected to establish a baseline. Afterwards, over the course of a year, stool samples were collected monthly. Within the experimental cohort, additional stool, blood, and urine samples were collected upon the event of a UTI. In short- enough samples to try to see what was going on before, during, and after a UTI.
With this design, the researchers were able to answer some important questions. Remember, these initial questions are focusing on the association between the gastrointestinal microbiome and rUTIs.
What are some key gut microbiome characteristics of women who have rUTIs?
There is a depletion of butyrate producers and other helpful short chain fatty acid (SCFA) producers. There was also a decrease in bacteria varieties, also known as richness. These features are consistent in a variety of immune based disorders, check out these articles for the deadly combination. From the types of genera present, the researchers were able to deduce a functional loss of important intermediates in sugar degradation and biosynthesis of amino acids needed for a stable microbiome. Specifically, those that are important in maintaining the health of the community and sugar malabsorption in the host. None of these factors worsened prior to each UTI event; they were simply a universal feature specific to the rUTI cohort.

Do the same invading bacteria found in the urine also invade the gut microbiome?
The most common type of bacteria found in UTIs (82% of the time) is E. coli and usually called UPEC or uropathogenic- E. coli. Gastric infections like C. difficile or food poisoning favorite, Salmonella, typically occur in individuals whose microbiomes are low in richness and lack SCFA producers. As the rUTI cohort demonstrated these features the researchers wanted to see if this was the case for E. coli.
Overall, there were no overt differences in gastrointestinal E. coli abundances between the control and rUTI cohorts. But what about more subtle dynamics? There were incidents in the rUTI cohort where 10 times the normal amount of E. coli was observed in the gastrointestinal tract. Unfortunately, they were not reliable predictors of a rUTI event within the experimental cohort.
When researchers compared various strains of E. coli in urine and stool samples, they observed two phylotypes or similar enough genetic elements that were characterized as “persistent” and had “virulent” characteristics. Exciting news! That is until they see that these strains were present in both control and experimental cohorts. The researchers think that the genetic information may not be sufficient to provide insight as to why some E. coli strains change their behavior or activate virulence factors and why this doesn’t happen in every case and in future experiments should collect more urine samples.
Is the antibiotic treatment effective or is it harming the host and maintaining an unstable community composition?
Antibiotics were only able to temporarily eradicate the pathogenic microorganism as after a period, the bacteria would appear again in stool and urine samples. On the flip side, the same strain occurrence, where the same culprit would trigger UTI symptoms repeatedly, occurred in only one participant. But the strains that did reoccur were similar enough to the former infecting strain. Moreover, the pathogenic strain would simultaneously appear in stool samples. This indicates some sort of migration of these strains between the anus to the urinary tract.

Regarding community dynamics, this was not clear as there were no significant differences between antibiotic consumption and changes in community composition. The worsening of low community diversity and instability after antibiotics did not last more than 2 weeks. Essentially, a longer observation period would be needed to determine if repeated antibiotic exposure incrementally drives an even more unstable bacterial community composition. Nevertheless, it is always worthwhile for researchers to explore potential treatment options other than antibiotics (link to bacteriophages paper).
Are there host features contributing to the likelihood of having a rUTI?
As the gut bacteria do not exist in vacuum and the individual may have something that makes them more susceptible to rUTIs, the researchers explored the immune system of the participants. By looking at peripheral blood mononuclear cells (PBMCs) participants obtained from blood samples no major differences in the immune system were seen between the two cohorts.
The researchers observed which genes were “active” in these PBMCs within the two cohorts. Only two genes that were heavily expressed showed up in the RNA-seq assessment. ZNF266 which is linked with an overactive bladder and inability to control pee in women. A non-coding version of LINC00944 was detected to be linked with propagating an inflammatory reaction. A loss of this gene means an absence of the important natural killer cells that would have suppressed a bacterial invasion.
However, the authors made no additional comparisons between composition and gene regulation. They simply highlighted the immune regulatory differences between the cohorts potentially impacting the gut microbiome.
Some things to keep in mind
While some of the insights made from the results of this study were interesting, this was an initial exploration of the gastrointestinal microbiome and rUTI relationship. Though some crucial elements were neglected in the study. For example, the vagina has its own microbiome, but no vaginal swabs were taken. While many pathogenic bacteria are derived from the rectum- this is problematic as a third of bacteria in rUTI are unique to the vaginal microbiome. They were also not able to clearly establish whether long-term antibiotics maintained the skewed population of bacteria unstable composition and directly led to UTI. The researchers were only able to superficially delve into links between the gut microbiome and rUTI, but they did start laying the groundwork to study the relationship.
Link to the original post:
Featured image: Created by Author in Bio Render