Breaking down the microbiology world one bite at a time
Ebola Virus Sneaks Out Through Cellular Tunnels
Written by
Neelabh Datta
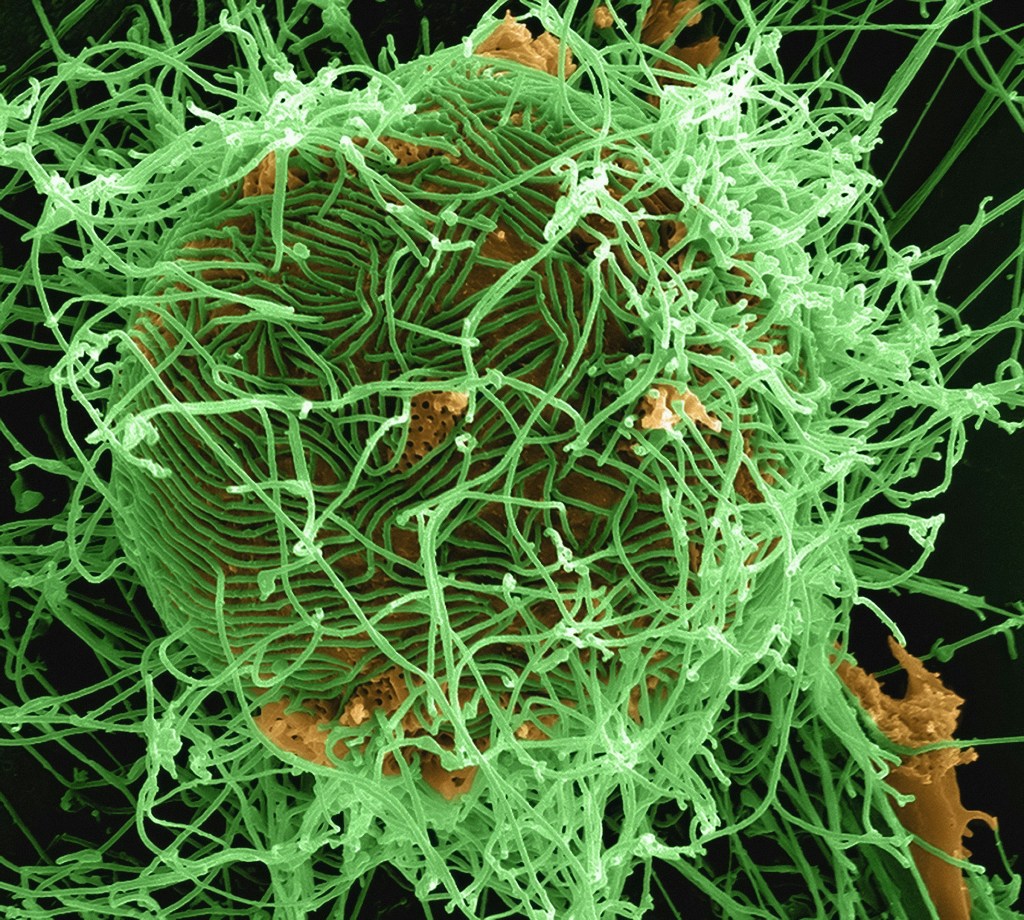
Ebola virus (EBOV) is a deadly RNA virus that causes hemorrhagic fever in humans and animals. It infects macrophages and other cells by macropinocytosis, a process of engulfing extracellular fluid and particles. The virus has a negative-strand RNA genome that is encapsulated by several viral proteins which mediate various steps of the viral life cycle, such as entry, replication, transcription, nucleocapsid assembly, and egress. To spread efficiently, EBOV exploits tunneling nanotubes (TNTs), which are long and thin cytoplasmic extensions that connect two cells and allow the transfer of cellular materials. TNT-mediated viral genome transfer can also increase the range of host cells that can be infected, leading to more severe disease outcomes. In a recent 2023 research, Djurkovic et al., demonstrated a novel mechanism of EBOV spread that involves these specialized nanotubes formation and nucleocapsid transport, in addition to the conventional release of virions from the cell surface.
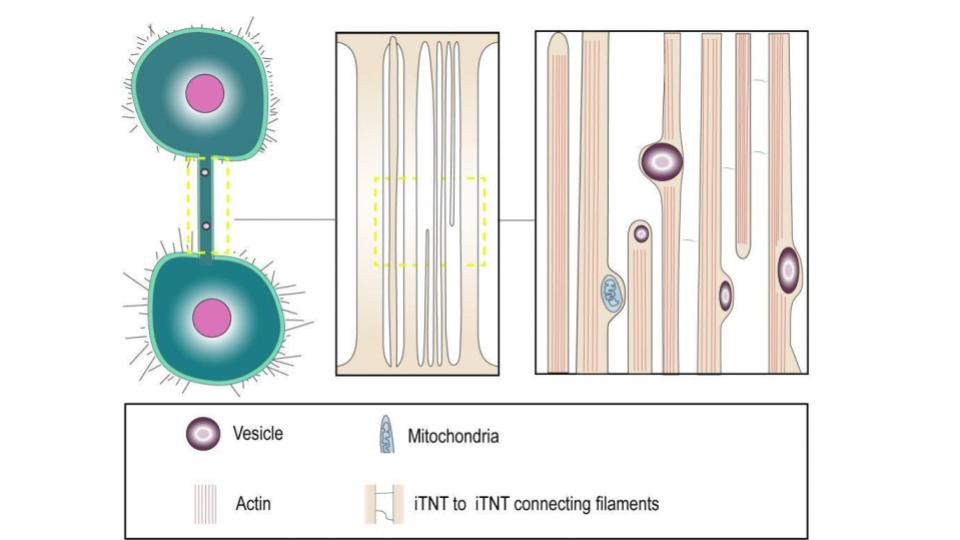
They found that only the TNTs that had tubulin and carried cellular organelles, also had viral components, such as proteins and RNA which the EBOV could transfer between cells, without causing infection. EBOV could resume its infection in cells that were treated with drugs that block virus entry, implying that the virus could replicate without entering the cells. EBOV-infected macrophages had higher ATP levels, indicating that the virus might use or alter mitochondrial functions for its advantage.
EBOV interacted with mitochondrial complexes and was found located near mitochondria in infected cells, which could provide energy for the virus replication cycle, such as making and releasing new viral particles [3]. The researchers also reported that EBOV prevented the activation of the mitochondrial antiviral signalling protein pathway, which is essential for immune response, by producing type I interferons. They explained that EBOV did this by interfering with the binding of viral RNA to the RIG-I receptor and affecting mitochondrial dynamics as well [4].
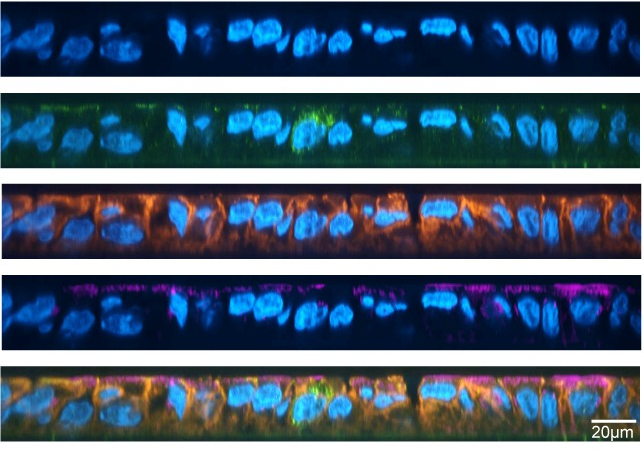
EBOV-infected macrophages were stained with antibodies which bind to proteins such as the matrix protein (VP40), viral glycoprotein (GP) and viral nucleoprotein (NP), which are involved in virus particle assembly and egress. VP40 and GP were detected in some TNTs connecting infected cells, but not always in the same nanotubes. Viral genomes associated with NP protein were also detected in the nanotubes, indicating that viral genomes could move within the nanotubes induced by EBOV infection. So it was hypothesized that EBOV VP40 and GP would interact with the nanotubes.

The researchers also observed that EBOV genetic material could induce and travel through the nanotubes without infection. This has important implications for EBOV persistence and re-emergence, as it is known that EBOV RNA can remain in immune-privileged tissues, such as the eye, the brain, and the testes, for a long time after recovery and cause a new infection with a similar sequence. It was speculated that the nanotubes could facilitate the movement of EBOV RNA within these tissues, as nanotubes are involved in intercellular communication and transport in the brain and the eye.
They found that these viral structures were distributed along the nanotubes and moved into adjacent cells. This allowed them to conclude that EBOV genetic material can trigger the nanotubes formation and move through them in a virus-independent manner. The efficacy of existing anti-EBOV drugs, which target GP were tested, and found that they could not prevent EBOV replication or spread in macrophage cultures via nanotubes. This indicated that EBOV can evade these drugs by using a virus-free mode of transmission and that it can persist in some tissues that are inaccessible to the drugs.
The researchers used the EBOV-GFP complex to infect primary human macrophages. They were interested in observing the formation of nanotubes that were positive for infection but did not affect cell survival after 24 hours. 24h is the time needed for one viral genome replication cycle. The infected cells were stained with fluorescent phalloidin stain and antibodies to detect F-actin, viral NP, and TNFAIP2, a host protein that is essential for TNT formation and localization. They found that the TNTs contained both TNFAIP2 and viral NP and were free-floating in the medium.
They also showed that macrophages could form TNTs with human umbilical vein endothelial cells, which are used to model endothelial infection by EBOV. This indicated that macrophages could facilitate the transfer of the virus to different cell types via TNTs, increasing the cell infection. The TNFAIP2 protein was also associated with TNTs in EBOV-infected macrophages. The authors hypothesized that EBOV infection creates a proinflammatory environment that favours TNT development, as evidenced by the upregulation of several cytokines that are linked to inflammation and mortality. Moreover, the researchers observed that viral genomes were transported along TNTs, in macrophages infected with EBOV. This observation was also seen in other similar types of filoviruses that induce inflammation, suggesting that TNTs may play a role in filovirus dissemination and pathogenesis by shielding the virus from immune detection and avoiding host defences. These findings presented in this paper enhance the knowledge of EBOV replication and provide new avenues for antiviral intervention.
Additional Readings
Feldmann, H., Sprecher, A., & Geisbert, T. W. (2020). Ebola. The New England journal of medicine, 382(19), 1832–1842. https://doi.org/10.1056/NEJMra1901594
Batra, J., Hultquist, J. F., Liu, D., Shtanko, O., Von Dollen, J., Satkamp, L., Jang, G. M., Luthra, P., Schwarz, T. M., Small, G. I., Arnett, E., Anantpadma, M., Reyes, A., Leung, D. W., Kaake, R., Haas, P., Schmidt, C. B., Schlesinger, L. S., LaCount, D. J., Davey, R. A., … Krogan, N. J. (2018). Protein Interaction Mapping Identifies RBBP6 as a Negative Regulator of Ebola Virus Replication. Cell, 175(7), 1917–1930.e13. https://doi.org/10.1016/j.cell.2018.08.044
Cárdenas, W. B., Loo, Y. M., Gale, M., Jr, Hartman, A. L., Kimberlin, C. R., Martínez-Sobrido, L., Saphire, E. O., & Basler, C. F. (2006). Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. Journal of virology, 80(11), 5168–5178. https://doi.org/10.1128/JVI.02199-05
Rustom, A., Saffrich, R., Markovic, I., Walther, P., & Gerdes, H. H. (2004). Nanotubular highways for intercellular organelle transport. Science (New York, N.Y.), 303(5660), 1007–1010. https://doi.org/10.1126/science.1093133
Delage, E., Cervantes, D. C., Pénard, E., Schmitt, C., Syan, S., Disanza, A., Scita, G., & Zurzolo, C. (2016). Differential identity of Filopodia and Tunneling Nanotubes revealed by the opposite functions of actin regulatory complexes. Scientific reports, 6, 39632. https://doi.org/10.1038/srep39632
Featured image: NIAID https://www.flickr.com/photos/niaid/14674486019/