Breaking down the microbiology world one bite at a time
Skin Microbiota Defends Colonisation By Candida auris
You might have heard of yeasts. They are the popular fungus used in leavening the dough for bread and other bakeries, but are also infamously known to cause skin infections. One such yeast is Candida that causes candidiasis. This genus comprises the largest medically important yeasts responsible for human infections with over 200 species! Among these species, a few stand out for their impact on our global health system, and this article chooses to target Candida auris, the emerging multidrug-resistant fungal pathogen.
Candidiasis and its repercussions, in brief…
CDC defines candidiasis as the fungal (yeast) infection that occurs when the generally quiet Candida living on the skin and in our bodies, grows out of control and flourishes (negatively) in or on our body.
In the first-ever list of health-threatening fungi released by WHO in 2022, Candida auris takes the second place. In fact, the report mentions that the overall mortality of invasive candidiasis ranges from 29% to 53%. In a study mentioned by CDC, candidemia (the most common form of invasive candidiasis) was found to have a mortality of 19–24%.
Analysing Candida auris to combat strategies against it
Considering everything so far, it is needless to stress why we need to combat the invasion of this fungus to subdue it to its normal commensal (harmless) state.
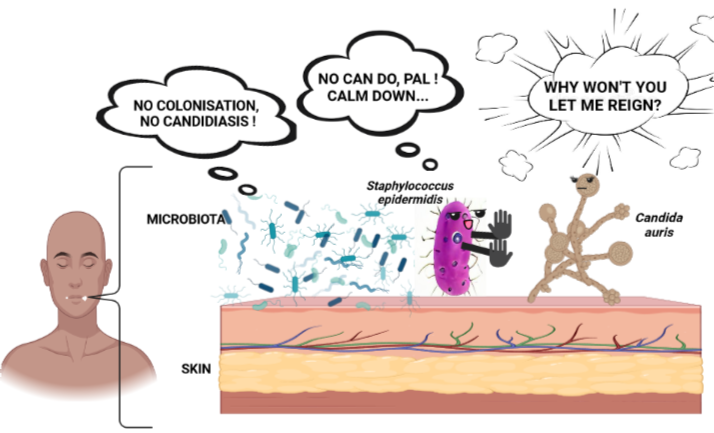
But first, we need to understand the inner workings of this fungus and its interactions. And this was done by Brooke Tharp and her team (2023), where they explored the role of skin microbiota that enabled the colonisation of Candida auris. They emphasise the importance of this study by stating that, “Understanding microbiota, C. auris, and host interactions in the skin is important to develop microbiome-based therapeutic approaches to prevent and treat this emerging fungal pathogen in humans.”
Normally, commensals prevent pathogens from “being cosy” by either secreting molecules or stimulating skin immune factors, interactively. This impacts the colonisation level and the degree of harm (pathogenesis) of these pathogens on the human skin.
The table below shows the varying species between the two sets of people (positive and negative) in both fungal and bacteria composition. This shows that C.auris even affects the host microbial composition and their responses!
| Microbial composition of patients | C.auris | |
| Positive (+) | Negative (-) | |
| Fungi | Candida species | Malassezia species |
| Bacteria | Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Providencia stuartii, and Morganella morganii. | Staphylococcus hominis, Staphylococcus epidermidis, Staphylococcus caprae, Anaerococcus nagyae, Peptoniphilus tyrrelliae, Anaerococcus octavius, and Corynebacterium tuberculostearicum. |
Among them, commensal microbes interact through immune factors to drive away pathogens. Effective communication is important for them to operate optimally.
Let’s consider two popular skin immune factors inhibiting C.auris: interleukin 17 (IL-17) and cathelicidin or LL-37. IL- 17 associates with T helper 17 cells to control yeast colonisation of the skin. LL- 37 is an antimicrobial peptide produced by human keratinocytes (outermost epidermal cell of the skin). It inhibits the fungus by prompting various surface changes on it, inhibiting its cell cycle, and inducing oxidative stress.
Scientists used a homologue (similar gene in another species) of LL-37 that is cathelicidin antimicrobial peptide (CAMP) expressed in mice. They found that the lack of CAMP and its regulating molecule — hypoxia-inducible factor 1 alpha (HIF-1α) transcription factor made the rodent’s skin susceptible to pathogens. And moreover, these two molecules are highly expressed in the intestine! There, they prevent C.auris to colonise by providing gut microbiota-mediated resistance to the yeast. While studies with intestinal colonisation of C.auris are quite known, those related to skin colonisation are not.
Impact and new portals
This work on skin colonisation is just the beginning and opens doors for new scope.
- Immune responses vary among Candida species and through interactions among the host commensals with every pathogen. This is why identifying and understanding the other commensal players varying from Staphylococcus epidermidis (the representative commensal bacterium used in the study and you can know about it here and here) in defending skin health is important. After all, studying a particular species- to -species interaction cannot be extrapolated to summarise microbe-immune cell interactions on/ in skin.
- C.auris positive patients have much less skin commensal microbes than their fellow C.auris negative. Expanding this work explores the role and mechanisms with which skin commensals defend C.auris from colonising for its nefarious purposes. The knowledge gained actively restores commensal population in C.auris positive patients to counteract the yeast attack naturally, and lead to developing new antifungals.
- Further, through this work, subsequent nosocomial (hospital- originated) transmission can be disrupted to fend off mortality owing to invasive candidiasis (via C.auris) (Remember the stats at the start?) .
- Right now, Staphylococcus hominis, which forms part of the skin microbiota, is already in human clinical trials to treat skin infections and eczema. This exemplifies how utilising commensal microbes to treat C.auris is not just empty talk, but is actually feasible.
All in all, extrapolating this work has a high potential to tackle the health threatening C.auris. We hope to advance far ahead in addressing such global health concerns. The Chinese proverb: “A journey of a thousand miles begins with a single step” reminds us that this work is truly one of the stepping stones to control C.auris colonisation and consequently the resulting rampant candidiasis.
_______________
Original article: Tharp, B., Zheng, R., Bryak, G., Litvintseva, A. P., Hayden, M. K., Chowdhary, A., & Thangamani, S. (2023). Role of Microbiota in the Skin Colonisation of Candida auris. Msphere, e00623-22. DOI: https://doi.org/10.1128/msphere.00623-22
Feature image: Image source: Created by Tejaswini Petkar using craiyon.com and biorender.com.