Breaking down the microbiology world one bite at a time
Nitrogenase & Climate change: From past to future
Nitrogen, the plentiful component of air (78%), stays quite elusive in the atmosphere. Nitrogen is needed for survival and growth, and thus rises a need to capture it from the atmosphere to a soluble (usable) form that travels across various sources and organisms before returning to its original source— the atmosphere, through the nitrogen cycle (image 1).

Capturing nitrogen from atmosphere to soil is called nitrogen fixation. This occurs naturally in two ways: lightning, and through a biological molecule, the nitrogenase, produced by a group of microbes called diazotrophs.
Nitrogenase
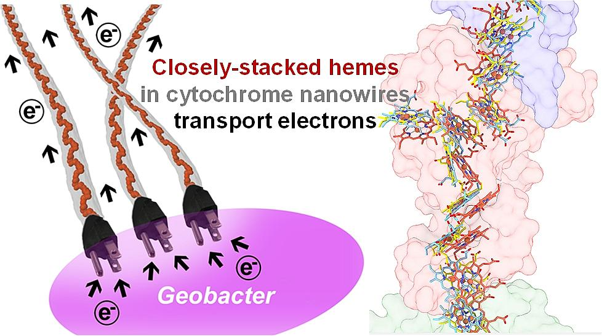
Nitrogenase, the only key enzyme for biological nitrogen fixation, is a fascinating biomolecule (see image 2). This metallo-enzyme has various forms. It uses iron-sulfur clusters that vary depending on the metals (molybdenum (Mo), vanadium, or only iron) they incorporate. All diazotrophs possess Mo-nitrogenase but only a small fraction harbor the alternative nitrogenases (vanadium nitrogenase and the iron-only nitrogenase).
Despite the importance and ubiquitous presence of nitrogenase, we do not know what exact conditions triggered its origin, nor its evolution with its co-metals that in turn wavered in abundance through time. Interestingly, this unique enzyme seems to originate 3.2 billion years ago before the Great Oxidation Event (GOE), a time where oxygen was barely making its debut in shallow marine environments.
Predecessors of nitrogenase could not fix nitrogen. They came from proteins resembling an enzyme called maturase which is generally involved in maturation and/or the processing of other molecules. These predecessors evolved as iron-sulfur clusters of the enzyme complex in the form of NifEN — a protein complex involved in the assembly of nitrogenase in the NifD active site. NifD is the core component of the enzyme where nitrogen binds and converts to ammonia.
It is speculated that early nitrogenases used molybdenum (Mo) before eventually incorporating vanadium or iron cofactors. The reason why Mo was selected over other metals despite its lesser availability before the GOE, was that Mo-nitrogenases require less ATP molecules, and catalyze the reduction to ammonium (NH3) at a much faster rate.

Sustenance of nitrogenase
Before delving further, it’s worth pondering how nitrogenase, an enzyme highly susceptible to oxygen, has sustained throughout the ages, particularly post- GOE (the Great Oxidation Event)…
- In the marine world
In the marine environment, nitrogen dominates in two major forms: gaseous N2 form (94%) and soluble and fixed nitrate form (5.3%). Since the bioavailable form of nitrogen is very less, diazotrophs play an essential role in nitrogen fixation, with the cyanobacterium Trichodesmium (∼ 60 − 80 %) dominating. Now, how does this aerobic cyanobacterium protect its nitrogenase from oxygen?
Trichodesmium species lack heterocysts present in other cyanobacteria. Instead they have diazocytes — surrounding cells that protect the nitrogenase. These cells lack the glycolipid layer surrounding heterocysts that allow increased gas into the cells, and therefore the enzyme remains gas-free.

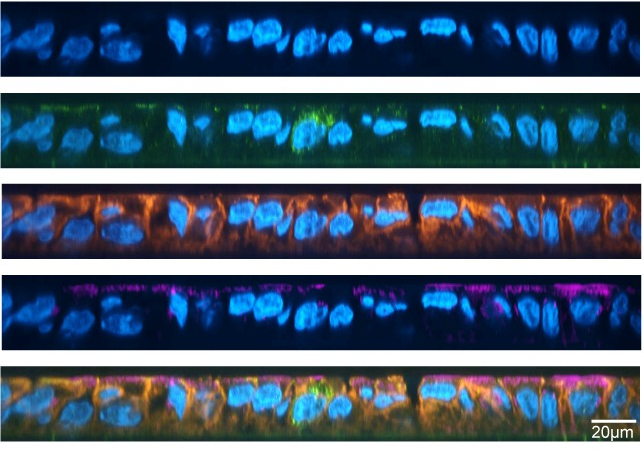
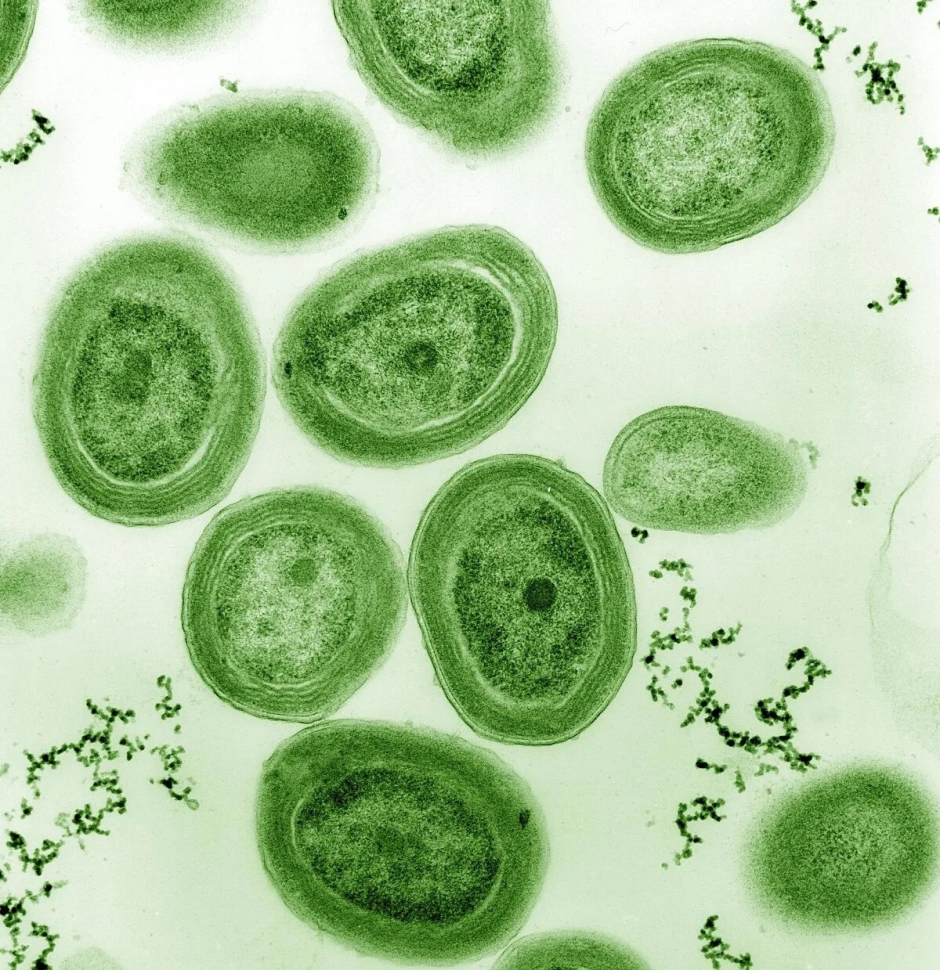
Furthermore, Trichodesmium colonies (image 3) utilize siderophores from other bacteria in oceans with low iron content. In exchange, they provide a favorable microenvironment to dissolve iron for the enzyme despite high levels of Mo in the waters. An inclusive way for the other life forms to flourish, wouldn’t you agree?
- In terrestrial environment
In contrast inland, Mo is one of the scarcest metals and iron highly abundant. Terrestrial diazotrophs form 2 major groups: symbiotic heterotrophs and free-living. The former includes microbes like Rhizobia (image 4) and Frankia forming symbiotic relationships with plants like legumes when nitrogen availability in the soil is low. They are therefore much more studied, especially due to their significance in agricultural sciences. We however do not know much about the free-living diazotrophs, as they are responsible for nitrogen fixation in cold and arid environments with low vegetation, despite having alternative nitrogenases.
As we study how evolution has shaped nitrogenase in the past, we hope to predict its future impact on us and our planet…

Climate changes and what does it mean for the future of the nitrogen cycle…
Since biogeochemical cycles sustain life, the drastic climate changes occurring worldwide certainly influence the nitrogen cycle. While some arguments lean towards a positive outlook, others do not look as favorable.
Increasing CO2 levels, for instance, mean increased photosynthesis and higher nitrogen demand for the process. In fact nitrogen fixation has the potential to enhance by 29% in diazotrophs ! But some studies have found that despite this, the high CO2 does not have much impact. Only 3% of the cell energy and even lesser growth rate of the diazotroph (such as Trichodesmium) could be affected.
In the oceans, increased CO2 means ocean acidification and easy iron solubilisation. That leads to less iron available to Trichodesmium and similar diazotrophs, in turn negatively altering their metabolism. This could have disastrous consequences considering Trichodesmium species are the major nitrogen fixers in marine environments.
Increasing temperature, owing to climate change, is another important influencer. They have a stimulatory effect on the diazotroph… well…at least until it reaches their “thermal maxima”, that is the temperature at which the organism can no longer withstand heat stress. With increasing global warming, a better response is expected in tropical areas over temperate ones, as most species have a higher optimal temperature ranging between 29°C and 37°C.
In reality, however, for the marine Trichodesmium and Crocosphaera which already live in warm waters (~30°C) with a thermal maxima of 36°C, rising temperatures would directly threaten their existence, and by extension affect the nitrogen cycle in marine ecosystems! But there many other nitrogen- fixing species with their unique requirements and responses to the changing climate, and therefore the scientific group’s statement gets emphasized: “ it is becoming increasingly important to develop more complex climate models to incorporate the variety of responses that biological nitrogen fixation may exhibit in response to the changing environment ”.

To conclude…
Understanding how the nitrogen cycle, particularly the nitrogenase, is impacted by climate change is crucial for our planet and all living things. So, as the researchers claim , we need to know how “nitrogen fixation originated, persisted, and proliferated across diverse ecological niches over billions of years.” To do this, they suggested an integrated approach from genomic databases, analyzing ancient protein sequences through evolutionary and in silico models, to laboratory studies.
This will not only patch the gaps in our geochemical record, but also unveil various ideas and innovations to tackle future ecological and climate challenges by digging from the past…
Link to the original post: Rucker, H. R., & Kaçar, B. (2023). Enigmatic evolution of microbial nitrogen fixation: insights from Earth’s past. Trends in Microbiology. DOI: https://doi.org/10.1016/j.tim.2023.03.011
Featured image: Ft nitrogenase as reporter on climate change. Image source: Original image by author using biorender.com, wikipedia.com, bing.com and freeSVG.com