Breaking down the microbiology world one bite at a time
Flu’s Trojan Horse: Hijack an Iron Transporter for Cellular Invasion
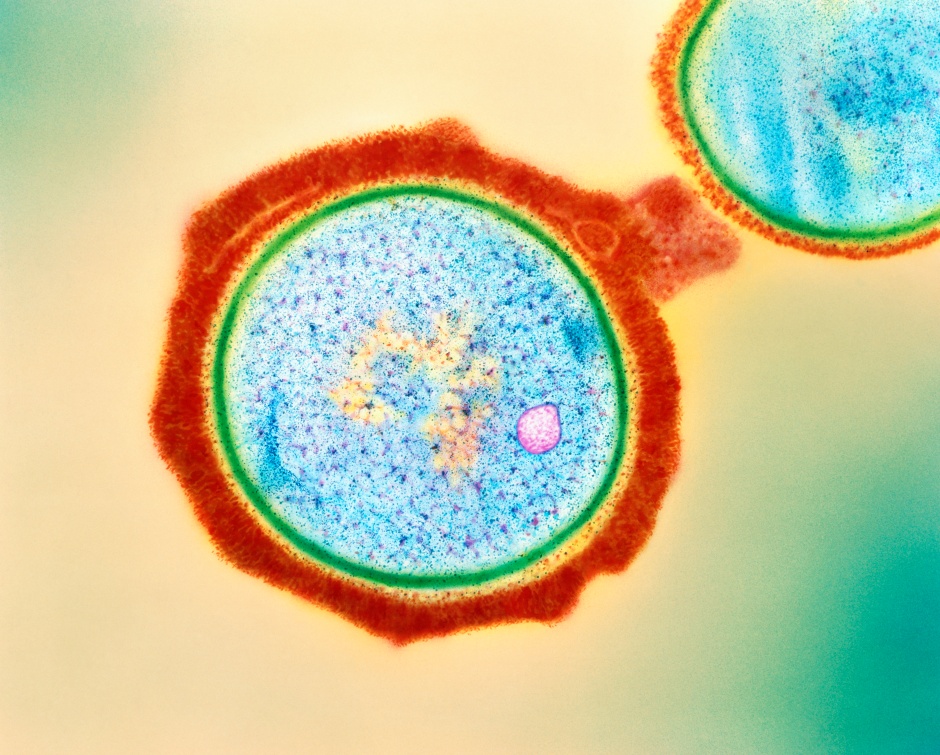
Flu is a highly contagious disease caused by a type of respiratory virus called Influenza A virus (IAV) that caused global pandemics. This virus raises considerable concern due to its capacity to induce severe illness and hospitalization, particularly in vulnerable demographics like the elderly. As a result, this disease has placed a significant burden on the healthcare system and the general public during seasonal outbreaks. Given that the spread and replication of IAV depend on the infection of its hosts such as humans, vaccination has served as an effective preventive measure to curb IAV transmission. Nevertheless, despite the acknowledged impact of IAV, the precise mechanism by which it enters into host cells in the course of infection is still not fully understood. A recent study published by researchers from University of Geneva, Switzerland, shed light on how IAV can enter into host cells by exploiting a human protein linked to iron metabolism.
Mining the host factor that allows IAV to enter into host cells
During infection, the flu virus (IAV) infects host cells through a two-step process: binding and entry. To attach to the cellular surface, IAV utilizes a biomolecule known as glycoprotein, which is composed of proteins and carbohydrates (sugars). Specifically, this viral glycoprotein, hemagglutinin, binds to sialic acid, a sugar compound present on the surface of host cells. While the binding step of IAV is well-characterized, the specific host factor that can facilitate IAV entry remains largely unclear.
To look for the host factors that are essential for IAV to enter into cells, the study group leveraged a technique called proximity ligation assay to capture all proteins that interact with hemagglutinin in human lung cells. Then, the researchers took a biochemical approach where they used an analytical tool, mass spectrometry, to precisely identify all the hemagglutinin-interacted proteins. After carefully analyzing the results from multiple experiments, the research team focused on the proteins present on the cellular surface. Therefore, they identified transferrin receptor protein 1 (TfR1) as the primary candidate for further investigation.
Recycling of iron transporters allows IAV entry into cells
TfR1 is a protein that plays a critical role in the delivery of iron from the blood plasma to the body. This protein forms a complex with another different protein known as transferrin that can bind iron. The import of iron is then mediated by endocytosis, a cellular process that accounts for the uptake of external substances into cells by “infolding” of the cellular membrane. During endocytosis, the TfR1-transferrin-iron complex is internalized into the cells, and eventually TfR1 protein will be brought back to the cellular surface by the end of the process. This repeating cycle mirrors the concept of recycling and ensures a steady flow of iron transport.
To test if TfR1 protein is required for IAV entry, the study group took both genetic and pharmacological approaches. For the genetic approach, the group genetically deleted the gene encoding for TfR1 protein in human lung cells, and cells lacking TfR1 protein have reduced IAV entry at the early stage of viral infection. Interestingly, the loss of TfR1 protein in cells did not change entry from other unrelated RNA viruses such as vesicular stomatitis virus (VSV), suggesting TfR1-mediated entry is IAV-specific. Consistently, increased IAV entry was also seen when TfR1 protein was overexpressed in cells. Lastly, the administration of a TfR1 inhibitor, ferristatin II, also blocks early replication of IAV in cells.
But how does IAV utilize TfR1 to enter host cells?
Using high-resolution microscopy, the researchers found both IAV and TfR1 share the same cellular location after viral entry into the cells. From this observation, the study group then hypothesized that IAV hijacks the endocytosis of TfR1 to gain access to host cells. To test this hypothesis, the team introduced different altered versions of TfR1 into cells , hindering their recycling. As expected, cells with defective TfR1 endocytosis showed reduced early replication of IAV, supporting the idea that disrupting the recycling of TfR1 can impede the virus’s entry into cells.

Importance of the study
Collectively, the findings from this study provide novel insights into the process of influenza virus A entry into cells, and pharmacological inhibition of TfR1 recycling can be a new potential therapeutic strategy to prevent viral transmission.
Featured image: Image is generated with DALLE 2