Breaking down the microbiology world one bite at a time
Breaking the Mold: A Yeasty Way to Fight Against Viral Infection
Antiviral Systems Vary in Diverse Organisms
Viruses are intracellular parasites that hijack host cellular machinery to support viral growth. While animal viruses like influenza and coronavirus have lately gained widespread attention due to their potential risks to public health, it is still worth noting that these tiny microbes can be found ubiquitously among a wide range of organisms. Therefore, the hosts of viruses are not solely limited to large organisms. In fact, some viruses can even infect microorganisms. For example, a virus called L-A mycovirus can infect Saccharomyces cerevisiae, a common yeast strain used for baking and brewing.
To counteract the invasion of pathogens, hosts have developed a myriad of strategies to mount an effective immune response during infection.
Higher-level organisms such as mammals have complex immune systems where antiviral response can be regulated by innate and adaptive immunity. Innate immunity is a broad defense against pathogens. For instance, in response to viral infection, cells secrete a protein called interferon to establish an antiviral state and inhibit viral replication. On the other hand, adaptive immunity can lead to the development of immunological memory and pathogen-specific immune responses. One of the effective ways to simulate adaptive immune responses is by vaccine administration.
In contrast, simple organisms lack the advanced immune system found in mammals. So how can less sophisticated organisms like yeasts still protect themselves from viral infections?
A recent work published in the journal PNAS discovered that yeasts, unlike other organisms such as mammals, have diverse and yet distinct antiviral systems to defend against viruses.

Source: https://www.mdpi.com/journal/pathogens/sections/Viral_Pathogens
Yeast Has a Versatile Toolbox to Combat Viral Infection
L-A mycovirus is frequently found in lab-grown baking yeast. Remarkably, despite chronic infection of L-A mycovirus, yeast growth remained unchanged under a standard culture condition. Therefore, using this specific virus as a model, a research group from university of Toronto identified several new antiviral systems in yeast with a genetic approach.
Previously two yeast factors were identified and known for their antiviral functions during L-A mycovirus infection. One is a mitochondrial protein called Nuc1, and the other is Ski3. Both of them can degrade viral nucleic acids.
In order to set up the experimental system, the study group first genetically suppressed expressions of NUC1 and SKI3 in yeasts (nuc1∆ ski3∆), and grew these cells in glycerol, a carbon source known to enhance mitochondrial activities in yeast. When these attenuated yeasts are grown in glycerol, L-A mycovirus infection will result in lethality and growth defects.
To mine for novel antiviral factors, the researchers first looked for genes that can interact with NUC1 via a published genetic database called BioGRID. Next, the team further selected candidate genes whose deletion can lead to growth impairment in yeast with nuc1∆background. If a chosen gene has a potential antiviral function, overexpression of this gene in nuc1∆ ski3∆ yeasts will block their growth defects in the presence of L-A mycovirus and glycerol. As a result, using this experimental system, the research group was able to identify several factors that can inhibit viral replication in yeast.
Interestingly, the novel antiviral genes found in yeasts from this work are involved in diverse cellular processes, and many of these genes have analogous copies in more sophisticated organisms including humans. For instance, HSF1, one of the antiviral genes identified in this study, also has a copy of HSF1 in humans. Strikingly, human HSF1 has also been reported to be associated with the replication and pathogenesis of diverse viruses including HIV, SARS-Cov2, and dengue viruses.
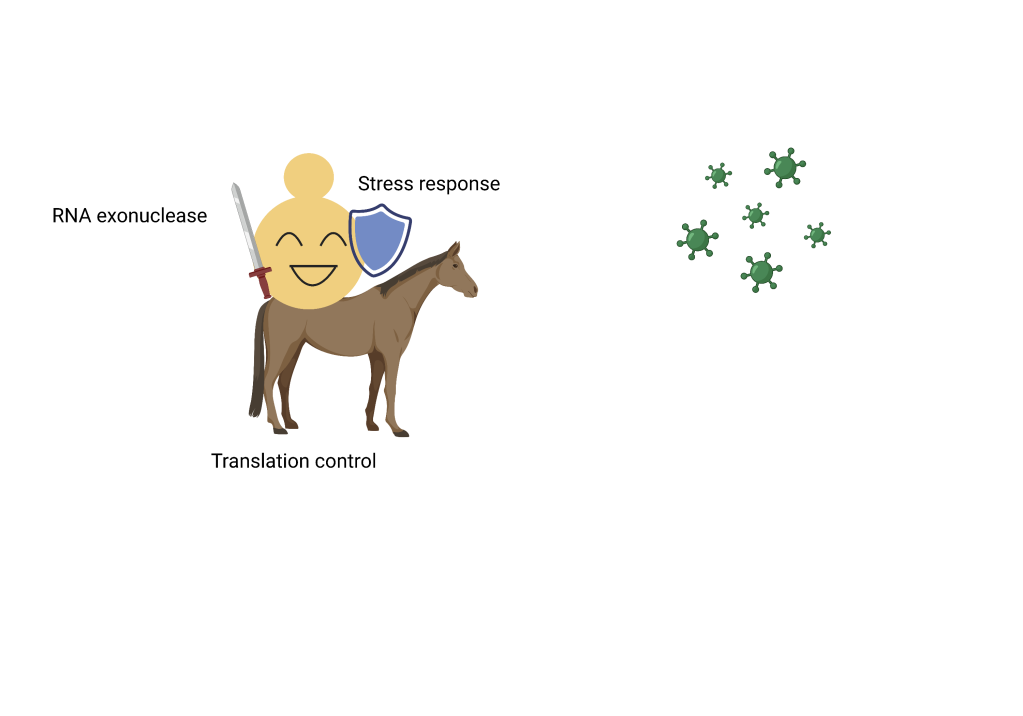
Therefore, by recognizing the evolutionary conservation of the antiviral genes found in this work, the researchers are able to gain insights into potential defense mechanisms linked to those genes. Given these newly discovered antiviral factors in yeasts are implicated in various cellular pathways, the study group subsequently classified these genes into specific antiviral systems as follows:
- RNA exonucleases: viral RNA can be degraded by this type of enzyme.
- Translation control: translation of host transcripts can outcompete ones from L-A mycovirus.
- Stress response: infection of L-A mycovirus can trigger a lethal stress response in yeasts. Under stress conditions like accumulations of toxic proteins, specific genes such as HSF1, a master regulator for cellular stress response, are induced to alleviate stress response.
The Implications of the Finding
While yeast lacks a complex immune system in humans, this single-celled organism is equipped with an array of arsenals for the antiviral response. Notably, the findings from this study highlight the diverse antiviral systems in yeast, and many of those newly discovered antiviral genes are also evolutionarily conserved in humans. Using both a genetic approach and a simple model organism, this work uncovers ancient immune responses that are critical for antiviral defense.
Link to the original post: Sabrina Chau, Jie Gao, Annette J. Diao, Shi Bo Cao, Amirahmad Azhieh, Alan R. Davidson, and Marc D. Meneghini. Diverse yeast antiviral systems prevent lethal pathogenesis caused by the L-A mycovirus. PNAS March 8 2023
Featured image: created using http://www.biorender.com
Additional sources:
https://www.nature.com/articles/nri3787
https://www.nature.com/articles/ni1153
https://febs.onlinelibrary.wiley.com/doi/pdfdirect/10.1002/2211-5463.13419
https://academic.oup.com/nar/article/34/suppl_1/D535/1133554?login=false