Breaking down the microbiology world one bite at a time
Using Green Bacteria to Engineer a Greener World
Imagine: a sweat-responsive shirt that ventilates or insulates depending on your needs. A patch that senses pathogenic bacteria in a wound and responds by secreting an antibiotic. A new biodegradable plastic, farmed from bacteria, that could transform the landfill landscape.
With the emerging field of engineered living materials (ELMs), these ideas have already leapt from sci-fi-like imaginings to experimentally-established reality: researchers take a microorganism, genetically engineer it to respond to a stimulus, and embed it in a bio-compatible material, allowing the material to sense and react to its surroundings. The response here is production of a useful protein performing a desired function – programming, with genetic code instead of computer code.
Responsive, pure-synthetic materials aren’t new; however, they are limited by the stimuli and the material’s properties. Contrastingly, living organisms are adaptable, able to respond in varied ways to many stimuli. ELMs push the boundaries of what bio-technological materials can do.
One recent Nature Communications paper demonstrates the ELM field’s potential to help environmental bioremediation efforts by creating a cyanobacteria-embedded material that breaks down a pollutant in response to a stimulus.
Why cyanobacteria?
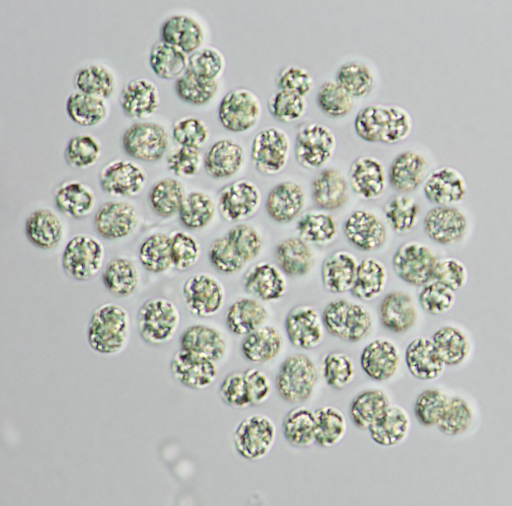
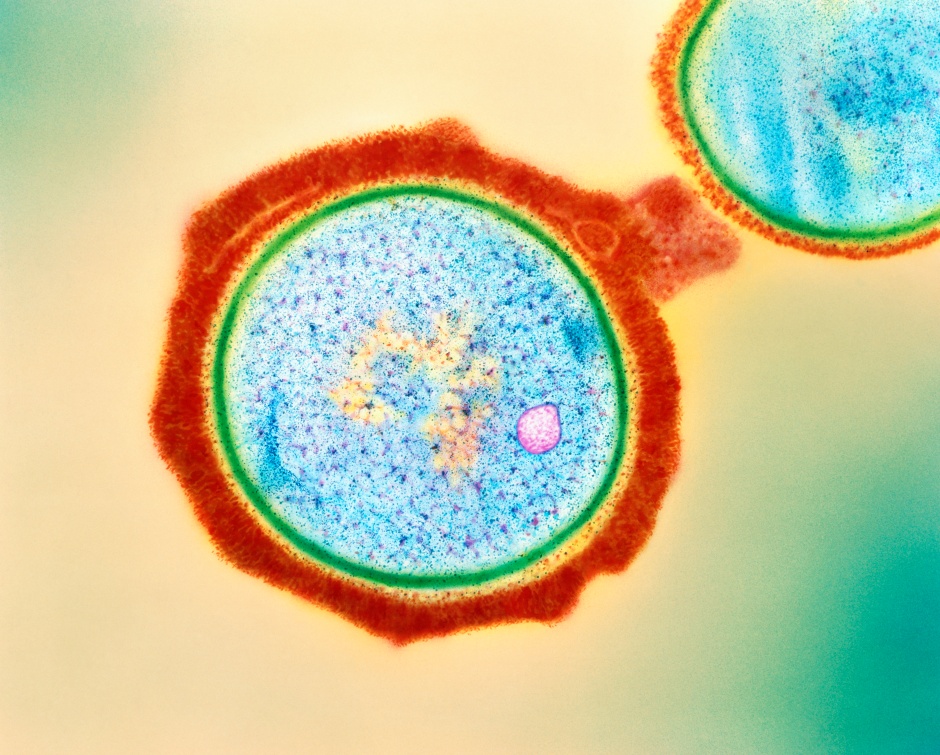
Cyanobacteria (Fig. 1) are single-celled photosynthetic microorganisms widely credited for the Great Oxygenation Event, an increase of Earth’s atmospheric oxygen 2.4 billion years ago that enabled the evolution of complex life. Residing in aquatic environments, cyanobacteria are important for the balance of many ecosystems.

While some of the over 2000 known cyanobacterial species cause toxic algal blooms, others are being explored as sources for biofuel or natural pigment production. Authors of this study chose cyanobacteria for their ELM because a) it was easy to grow at low cost; b) its required carbon source, carbon dioxide, is freely abundant; and c) many genetic engineering tools are already available for cyanobacterial research.
Study’s Goals:
- Design a stable 3D-printable material capable of supporting cyanobacterial life.
- Engineer cyanobacterial strains to produce desired proteins in response to a chosen stimulus, build an ELM with these strains, and show that the material can accomplish a desired task.
- Produce an cyanobacteria-based ELM that breaks down dye pollutant indigo carmine, and embed a controllable genetic ‘kill switch’ to show the potential for creating a bio-remedial ELM that can heal fragile ecosystems without further disrupting them.
Experiments and Results:
Step 1: Designing the Right Material
First, researchers selected a bio-compatible polymer for making their ELM hydrogel. Hydrogels are polymer networks that can hold large volumes of water without compromising structure, making them ideal for ELMs. After experimentation, authors chose alginate, a seaweed-derived polysaccharide. They determined the best combination of additional components to create a hydrogel with the optimal mechanical properties: viscosity low enough to not clog the 3D printer’s needle, but high enough that the ELM maintained shape; porous, so embedded cyanobacteria could spread, and transparent, to let in light for photosynthesis.
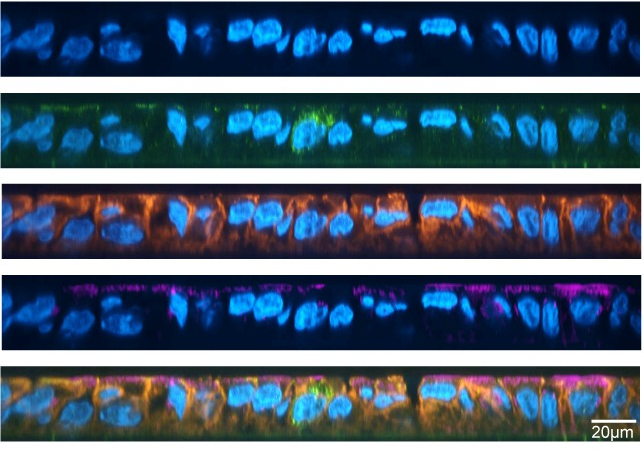
Researchers used direct-ink-writing, a versatile kind of 3D-printing, to build their ELM, printing their hydrogels with layers of live ‘bio-ink’. Tests determined their ELM’s properties – everything from what shape (Fig. 2) best supported cyanobacterial growth (a grid), to cyanobacterial survival over time, to ELM photosynthetic ability.

Step 2: Genetic Engineering + Initial Tests
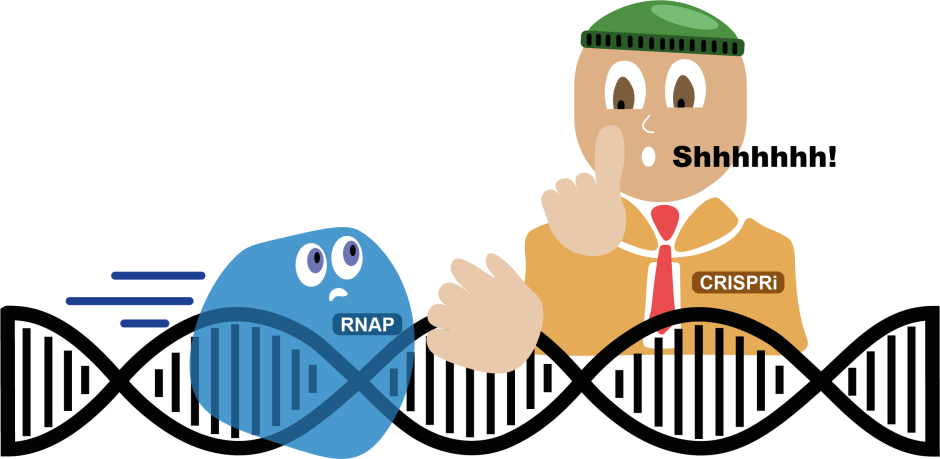
Some quick background: the ‘central dogma’ of biology is that every organism has a genetic code, DNA, which must be transcribed into intermediate molecule RNA, whose “message” is then “read” and translated into proteins, which carry out specific cellular functions. DNA is the “master code,” but not all genes are “on” all the time; cells can “switch” them on, leading to RNA transcription and protein translation, in response to environmental signals.
To “program” cyanobacteria, authors inserted a self-replicating circle of DNA into their cyanobacteria, Synechococcus elongatus. This DNA encoded a gene for a riboswitch-controlled fluorescent protein. Riboswitches are short RNA segments regulating gene expression in the middle of that “DNA → RNA → protein” process. Here, the DNA is transcribed into RNA, but can’t be translated into the protein until addition of a small activator molecule called theophylline, found naturally in tea. When researchers added theophylline to the ELM, it bound to the riboswitch, causing a shape change that allowed translation of the fluorescent protein.
Step 3: Creating an ELM that breaks down a pollutant

Researchers were now ready to design their pollutant-degrading ELM (Fig. 3). They engineered new S. elongatus strains, this time incorporating their new DNA sequences directly into the cell’s chromosomes. The DNA coded for Laccase, an enzyme capable of de-colorizing the dye pollutant indigo carmine, whose expression would always be “on.”
They also inserted a theophylline-responsive riboswitch just ahead of a gene already present in the S. elongatus DNA, whose over-expression kills the cell, allowing control of that gene’s expression – creating a kill switch. Experiments showed that Laccase was always being expressed, and that it could de-colorize indigo carmine (Fig. 4) – and they proved they could kill their ELM at will upon activating the riboswitch.

Takeaways:
Though further work is needed to create a waterway-ready bio-remedial ELM, this paper presents the first-ever cyanobacteria-based ELM using genetically engineered strains to achieve a specific goal, and it’s a major step forward in the field. This research shines light on how ELMs could help fight pollution, and inspires scientists to be creative and bold in imagining how new integrations of life and materials could innovate our future.
Link to the original post: Datta, D., Weiss, E. L., Wangpraseurt, D., Hild, E., Chen, S., Golden, J. W., Golden, S. S., & Pokorski, J. K. (2023). Phenotypically complex living materials containing engineered cyanobacteria. Nature Communications, 14(1). https://doi.org/10.1038/s41467-023-40265-2
Featured image: Image source: https://www.planetary.org/space-images/cyanobacteria-under-the-microscope