Breaking down the microbiology world one bite at a time
Multicellular bacteria
Bacteria are unicellular organisms, meaning unlike us they don’t have any organs or tissues, just one cell that keeps them alive. However, sometimes bacteria form multicellular aggregates, clusters of many cells.
But why would they suddenly adopt a multicellular form?
Most of the time they do it to protect themselves, usually by forming a biofilm, like a school of fish, where safety is in numbers, or hiding from harm. This biofilm is an aggregate of cells on a surface, which enables bacteria to increase their resistance to antimicrobials as they are more protected in the biofilm, as well as increasing immune evasion while giving them access to complex nutrients. They are working together towards a common goal to survive but are not completely cooperating as a single being., There is still a lot of competition happening between the cells in the biofilm
Figure 1. Biofilm formation: Stage 1, initial attachment; stage 2, irreversible attachment; stage 3, maturation I; stage 4, maturation II; stage 5, dispersion. From https://upload.wikimedia.org/wikipedia/commons/4/4a/Biofilm.jpg
For the biofilm to stay intact, the balance between competition and cooperation must be so that the entire community benefits by working together. One way they might balance this is to split their capabilities within the population, where one group of cells will perform some tasks while others will take care of other tasks.
What researchers still don’t know is when this phenomenon of sharing tasks is beneficial for the community and what it looks like.
In a new study, researchers have looked into the population of Vibrio splendidus (strain 12B01), and how sharing different tasks within this population affected the allocation of resources, growth, and reproduction.
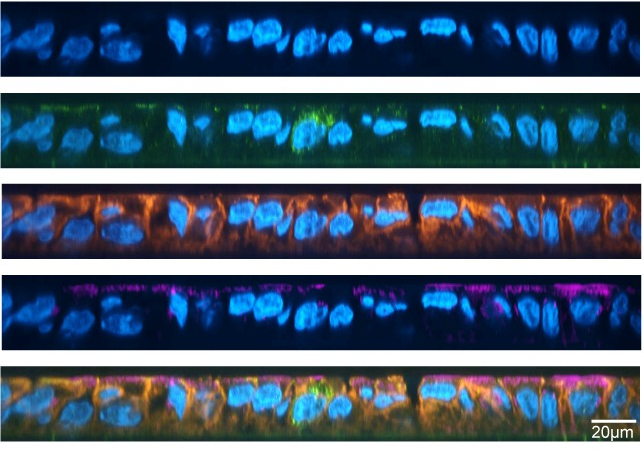
V. splendidus is a marine bacterium that can use a complex nutrient source to grow: alginate. Alginate is naturally present in the cell wall of algae. The researchers looked at how the different bacteria were spatially organised under the microscope as well as looking at what type of functions were performed within the different members of the community using RNA sequencing.
As the bacteria grew, the authors showed that two groups separated into two spatially distinct sub-populations to degrade the alginate more efficiently. They called the first population the “shell”, a static part of the community which surrounds the second sub-population which is more motile called the “core”. This core is able to store the carbon extracted from the alginate in case the bacteria run out of food later. These core cells are also actively reproducing, so younger cells tend to be in the center of the core.
Figure 2: “Life cycle” of the subpopulation of V. splendidus. In red the core population and in blue the shell population. Created with Biorender.com
They also saw that the two sub-populations were formed along a gradient of access to nitrogen, which is necessary for bacterial growth. Finally, they observed that once the population had too many cells in the core, the individual bacteria within the core would leave the population and form new clusters or biofilms on alginate, which could be considered the “birth” of a baby biofilm.
This study is the first to show a naturally occurring multicellularity where the partitioning of food and other resources leads to the emergence of a “life cycle”. The authors determined this life cycle where the young cells form the core and later become part of the shell part of the population. Eventually, This aggregate population will grow so large that it leads individuals of the core to form a new multicellular entity and begin the cycle again.
Link to the original post: Julia A. Schwartzman, Ali Ebrahimi, Grayson Chadwick, Yuya Sato, Benjamin R.K. Roller, Victoria J. Orphan, Otto X. Cordero, Bacterial growth in multicellular aggregates leads to the emergence of complex life cycles, Current Biology, Volume 32, Issue 14,2022 https://doi.org/10.1016/j.cub.2022.06.011
Featured image: Shutterstock
Originally published in The Microbial Times