Breaking down the microbiology world one bite at a time
Honey, I killed the bacteria.
By reading this sentence, you and I are communicating. We have developed a sophisticated means of communication, entailing verbal, and non-verbal cues. You can tell when someone is giving you good or bad “vibes”, as we have evolved the ability to perceive intention. Always listen to your gut! Communication is key to survival in all forms of relationships. While it may be obvious how humans and other mammals communicate, but how do microbes communicate?
Communication is a funny word… what does it really mean? Is it simply just giving off signals? Or is there a precise definition that we can follow? I like to think of communication as the exchange of information. Though this is a broad definition, I think it covers the main base. Computers thus communicate all the time, through the exchange of information in the form of codes and algorithms. Humans use speech, body language, and writing. So, what do microbes use?
Over the past 15 years or so, scientists began discovering a new way that microbes, and indeed almost every kind of cell, exchange key information – extracellular vesicles (EVs – more on what they are below – keep reading). The study of these EVs as we now refer to them, has recently become a very active area of scientific research across all domains of life.
Speaking of communication, what does this have to do with honey?
Well, I was getting there. But since you asked, I guess we can start now.
Recently, scientists based in Chile published a paper on the effects of Honey-derived EVs on the human oral microbiome. Specifically, honey EVs seemed to reduce the populations of two specific bacterial species Streptococcus mutans and Streptococcus sanguinis. If you didn’t know, S. mutans is the bacterium responsible for tooth decay (Loesche, 1986), whilst S. sanguinis is abundant in oral biofilms (Zhu et al., 2018), where it acts to change that environment to become less hospitable to plaque bacteria. Also of note, if S. sanguinis enters the bloodstream, it can become pathogenic (Zhu et al., 2018). Though that is cool on its own, the researchers delved a bit further into understanding why honey was having these effects.
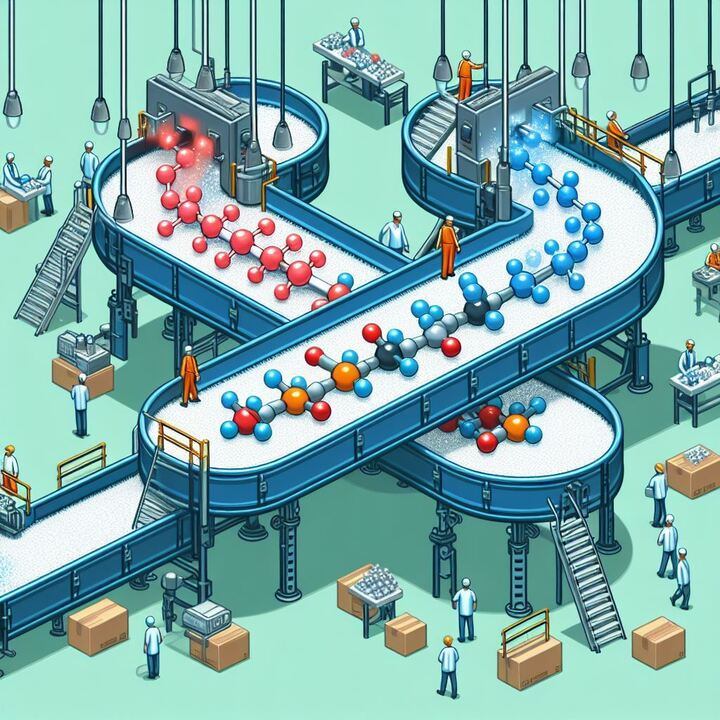
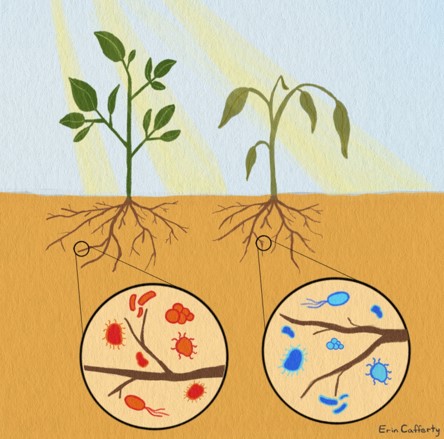
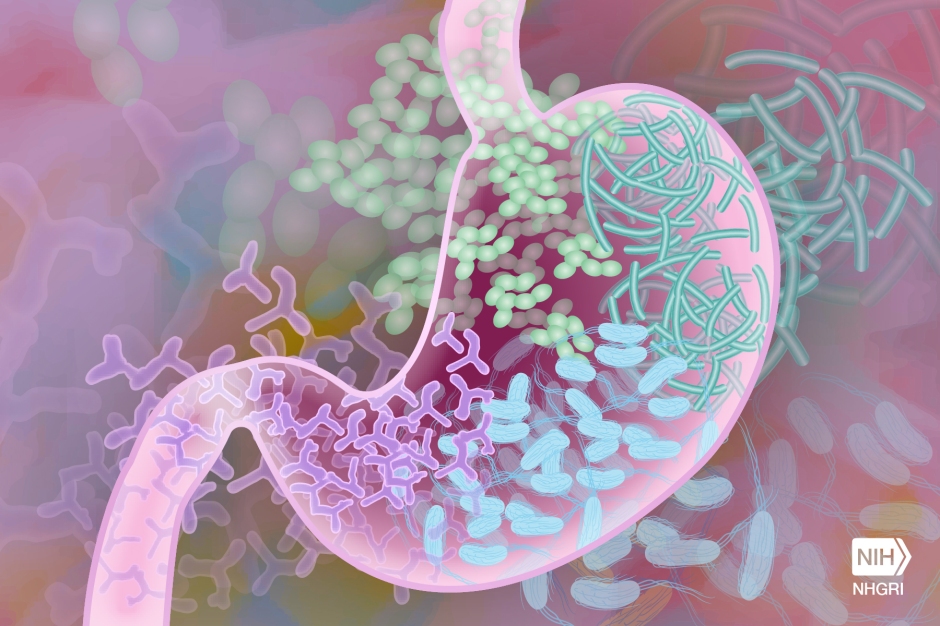
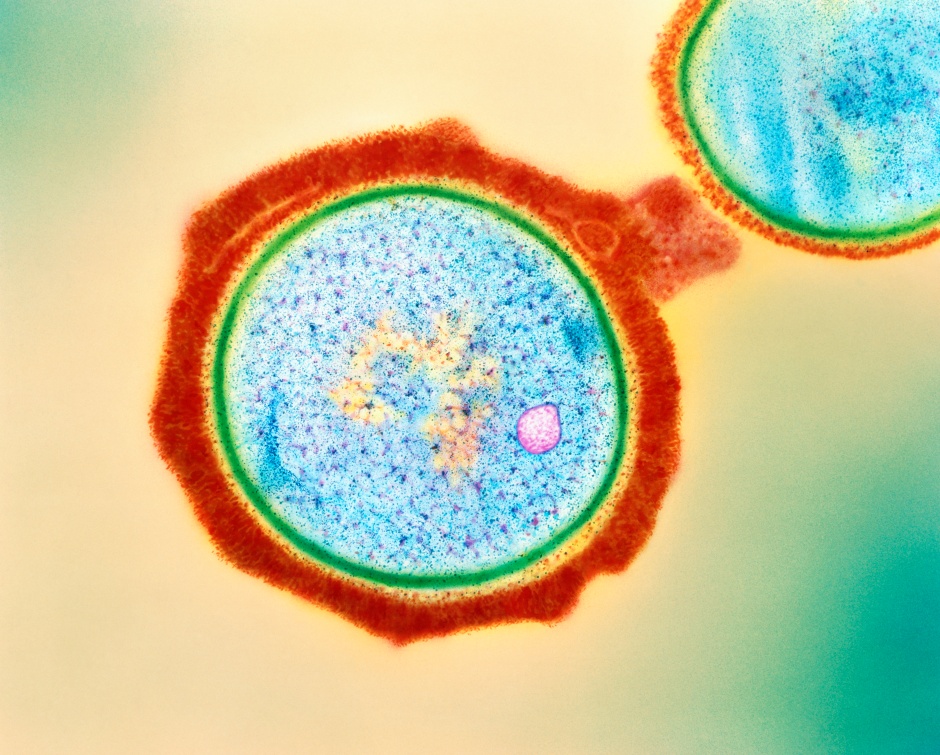
To understand what was happening, we need to first understand what an EV really is. The common definition of an EV is that they are small spheres that contain proteins, nucleic acids, and other cargos, that are released into the extracellular environment. Now, depending on when and how these small vesicles were produced, they can contain various amounts of cargo. Think about different proteins lining the membrane of the vesicle, or different molecules on the inside based on how and where these EVs were produced. That last part is critical – the ability to carry or store different materials on the inside of these EVs. Depending on the organism, or the environment in which they are produced, EVs can carry various materials (Figure 1).
So, let’s get to the good part. During their investigation, the researchers found that honey EVs contained antimicrobial compounds that included MRJP1, Defensin-1, and Jellein-3. Let’s dissect these three in order of appearance.
MRJP1: Major Royal Jelly Protein 1. This is a honeybee protein that is most abundant in honey. Researchers have reported that this protein has antibacterial effects against Gram-positive and Gram-negative bacteria. It can alter cell permeability and induce membrane lysis.
Defensin-1: this antimicrobial has a similar, but slightly different than the mechanism of action of MRJP1. It will induce pore formation, reduce membrane fluidity, and reorganise the membrane. These will ultimately cause bacterial membrane lysis.
Jellein-3: this is a small antimicrobial peptide that seems to be specific to Gram-positive bacteria. Surprisingly, not a lot is known about the antimicrobial activity of Jellein-3, or any of the other Jellein’s for that matter (Fontana et al., 2004).
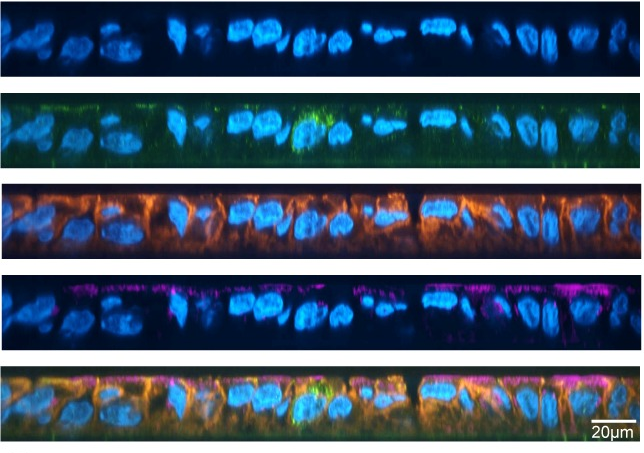
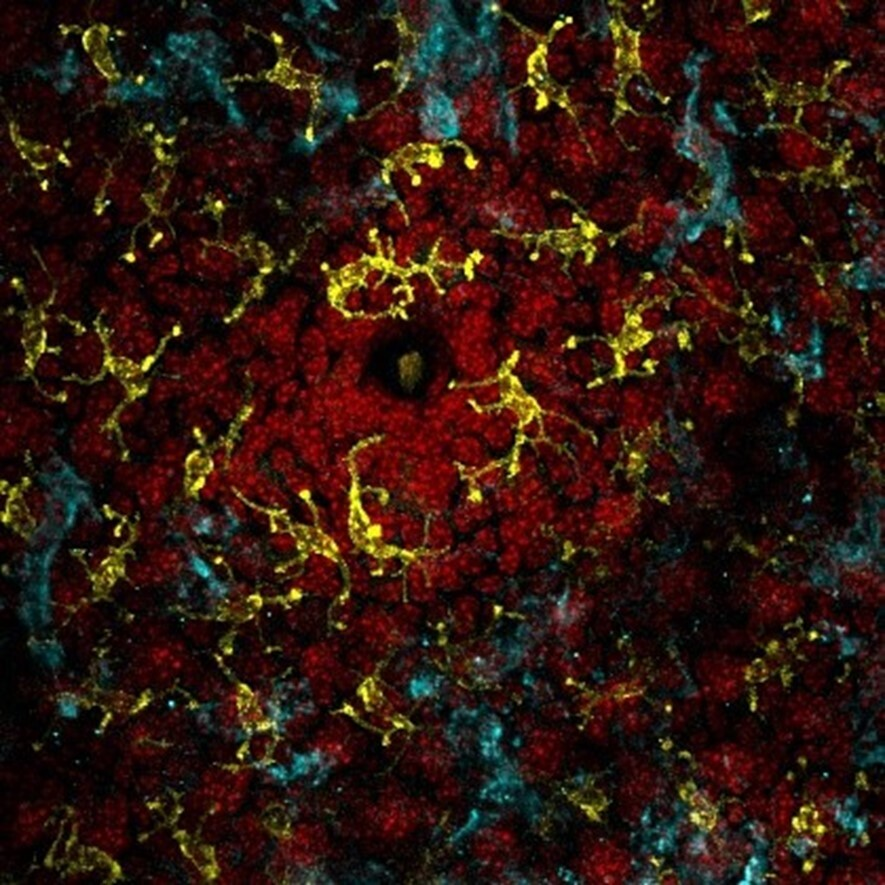
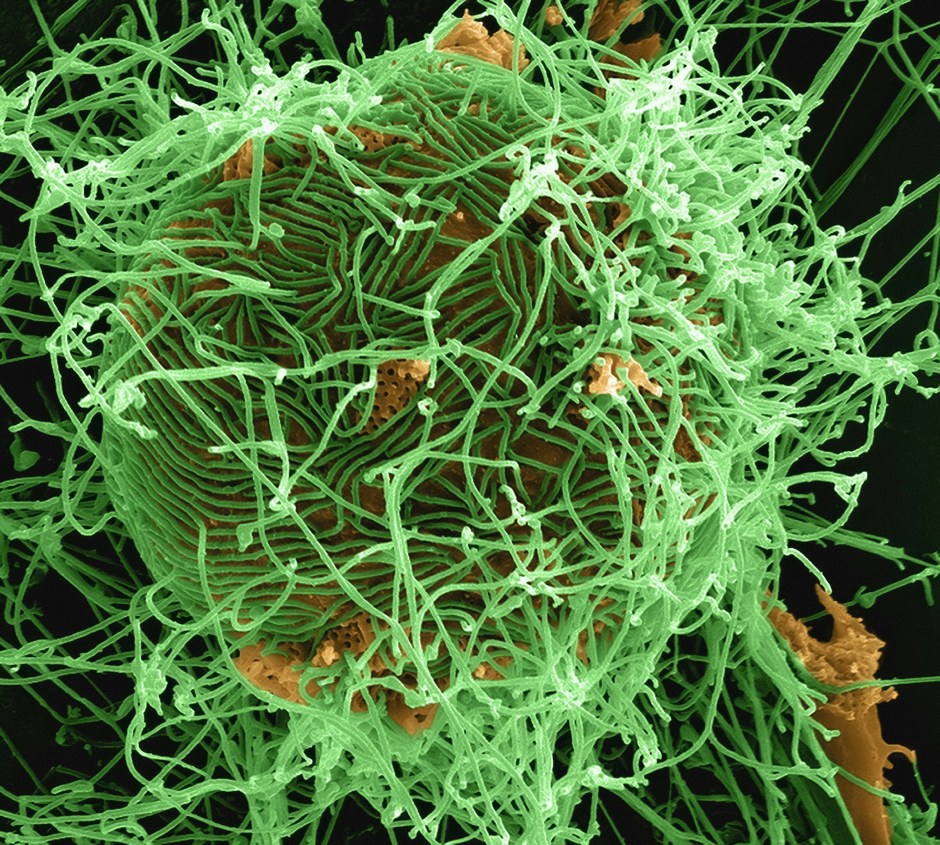
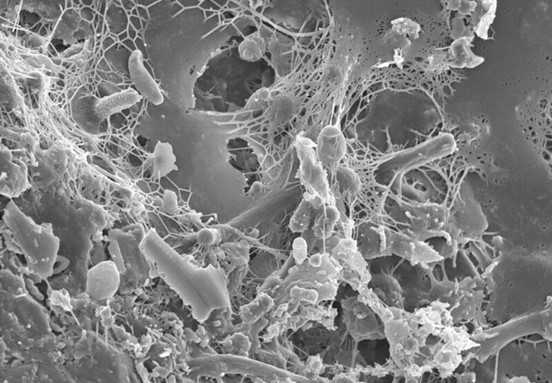
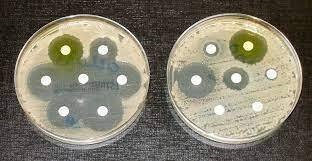
All three of these antimicrobial peptides were found in honey EVs. How did the researchers discover this? Well, they took actual honey and subjected it to ultracentrifugation (speeds of up to 30,000 rotations per minute). This separated the EVs from the honey, allowing the researchers to study the EVs in depth using a host of other techniques to identify the antimicrobial peptides. Using the isolated EVs, the researchers subjected the two oral bacteria S. mutans and S. sanguinis to experiments which observed the effect of these EVs on bacterial membrane integrity. They found that the bacteria which were exposed to the EVs had rigid, non-flexible membranes compared to the smooth and normal membranes of non-exposed bacteria. This change in membrane texture makes the rigid membranes more susceptible to rupture, thus confirming the antimicrobial effect of honey EVs on oral bacteria (Figure 2).
Cool! But why should I go tell my friends about this?
I want you to think about the movie Inception. In this movie, the ability to plant ideas into a target’s mind is explored through dreaming. Delving into deeper, and deeper levels of dreaming, the idea is that we can export an idea into someone’s mind, without them realising it’s a foreign idea. The same can be said of EVs. This study demonstrates that honey EVs contain antibacterial peptides. Whose to say we couldn’t purify these same EVs and load them with other antimicrobial agents? Is there a way where we can load EVs that contain the contents that we desire (spoiler alert – the subject of a future article). This is an active area of research, and it is quite exciting. The possibilities are endless.
References:
Zhu, B., Macleod, L.C., Kitten, T., and Xu, P. 2018. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol. 13: 915-932.
Loesche, W.J. 1986. Role of Streptococcus mutans in human dental decay. 1986. Microbiol Rev. 50: 353-380.
Huang-Doran, I., Zhang, C., and Vidal-Puig, A. 2017. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol Metab. 28: 3-18.
Fontana, R., Mendes, M.A., de Souza, B.M., Konno, K., Cesar, L.M.M., Malaspina, O., and Palma, M.S. 2004. Jelleins: a family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides. 25: 919-928.
Link to the original post: Leiva-Sabadini, Camila et al. “Antibacterial Effect of Honey-Derived Exosomes Containing Antimicrobial Peptides Against Oral Streptococci.” International journal of nanomedicine vol. 16 4891-4900. 20 Jul. 2021, doi:10.2147/IJN.S315040
Featured image: https://pixabay.com/photos/bee-honeycomb-pollinator-hive-4668171/