Breaking down the microbiology world one bite at a time
Attack strategy 101 of the debutant phage SI01
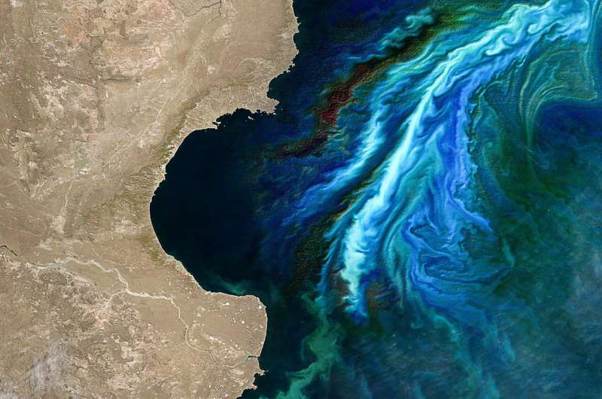
Did you know that algae drive 50% of the world’s oxygen production by photosynthesis? Algae earnestly collaborate with bacteria in oceans and form the key to navigate the marine carbon cycle. Bacteria generally supply inorganic nutrients enabling algal growth while the latter provide organic carbon for the bacteria. The constant interaction between the two, shaped either by themselves and/or the environment, is dynamic and intriguing, especially to those interested in learning the secrets of the seas and exploring marine biogeochemistry.
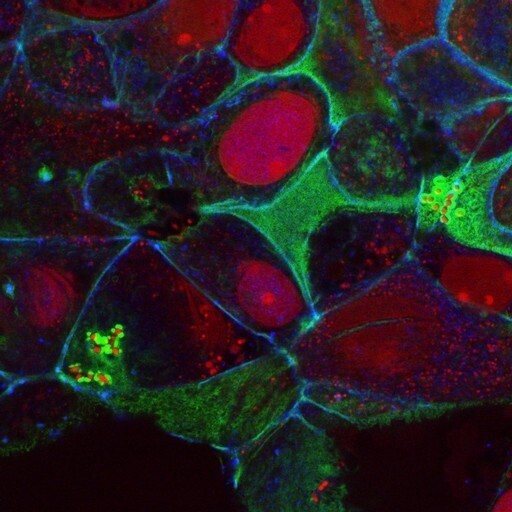
Not all algae look like scum on the surface of water; some are very beautiful to look at, especially through the ‘eyes’ of a microscope. Diatoms, for instance, are commonly known as “living opals”. They are the only algae with cell walls that are ornamented with intricate and striking patterns of transparent and opaline silica. Thalassiosira pseudonana is such a diatom and is favoured as a model organism for algal studies including interaction studies with other organisms like bacteria.

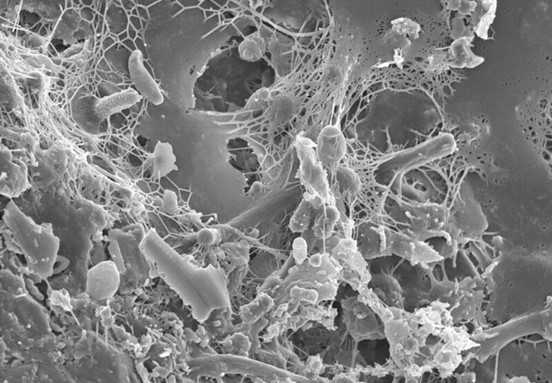
Like all algal-bacterial interactions co-existing since the start of evolution, the diatom Thalassiosira pseudonana associates with heterotrophic bacteria (bacteria using organic carbon as food) to form a biofilm.
Here’s a fun fact: Viruses have been estimated to lyse about 20% of the microbial biomass in the oceans daily. By lysing bacteria, they heavily influence the bacterial mortality rate by either eliminating them completely or in a regulated manner. Through this, the phages regulate the algal-bacterial interactions in biofilms- both directly and indirectly, since those very bacteria often negatively influence diatom growth.

Phage lysis centers in nutrient-rich areas surrounding algal cells that favour interactions between algae and bacteria, called phycospheres. Since the phycosphere has a higher microbial level than its surrounding water, it is more susceptible to lysis by viruses.
Interestingly, the algal phycosphere is frequently dominated by a few specific bacterial lineages. These bacteria actively form symbiotic chemical interactions with the host algae, thereby influencing the algal propagation.
In the phycosphere, the bacterial cells disintegrate when targeted by phages. This leads to an increase in both organic and inorganic nutrient levels within the zone. Consequently, the microbiota of the sea environment alters, as the non- (phage) infected microbiome gets boosted and the algae present now have more access to nutrients within this zone.

In 2022, a group of scientists in China explored the interaction among the three organisms: the diatom Thalassiosira pseudonana, the bacterium Stappia indica SNL01, and the phage SI01.
During their investigation, the researchers realised that among all the heterotrophic bacteria associated with the diatom Thalassiosira pseudonana, the bacterium Stappia indica SNL01 was the one tightly associated with the diatom. They found that it was this bacterium in particular that inhibited diatom growth by forming a biofilm. They were surprised to note that this recalcitrant bacterium could not be eliminated merely with mechanical and antibiotic treatments.
The bacterial genus Stappia is quite well-known for its unique and functionally important genes in its repertoire. For instance, the rhizobactin-like siderophore for iron scavenging was found to promote bacterial interaction with the algae.
In their study, the scientists isolated a new phage- SI01 in coastal seawater collected from Qingdao, China. They selected this phage for its ability to target Stappia indica SNL01. They found that the phage could counteract the growth inhibition by Stappia indica SNL01, by “lysing” or breaking down the culprit, subsequently preventing the biofilm to form. This eventually promoted diatom growth indirectly. Since bacterial and algal hosts are very much interlinked in biofilms, regulation by phages can thus profoundly affect the algal host (diatom) as well.
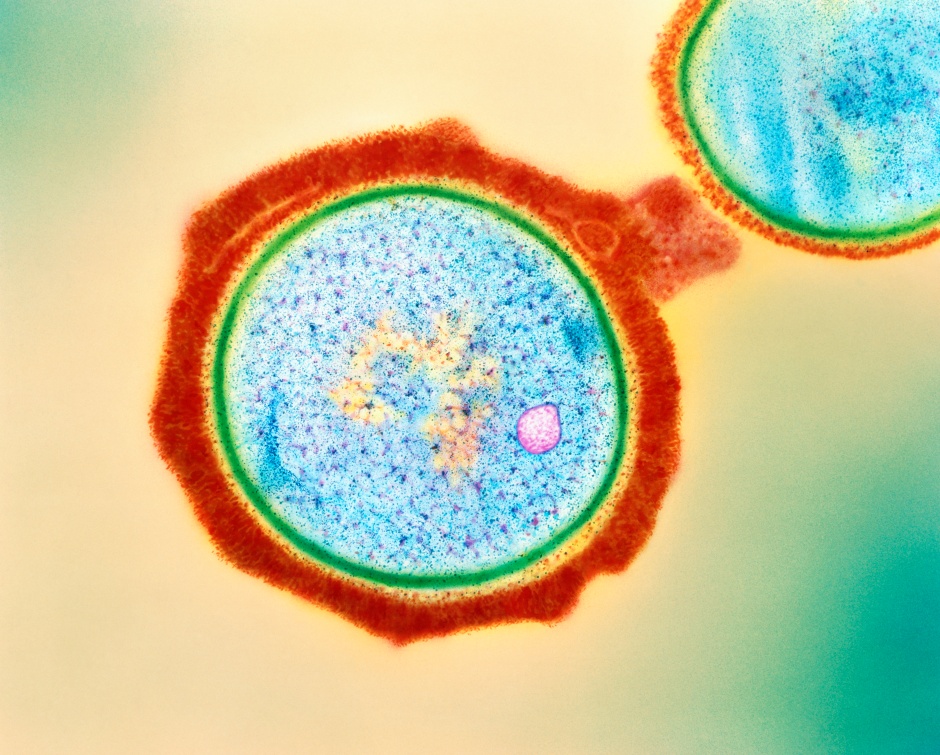
Phage SI01 is a new member of the virus family Podoviridae (detected by phylogenetic studies). Like all previous members of the Podoviridae, this debutant phage lacks lipids in its capsids. It harbours multiple lysis genes such as muramidase (to lyse cell wall), SleB (to lyse the outermost protective layer of dormant bacterial spores called cortex), and depolymerase-like tail spike protein (to facilitate binding and digestion of capsules by adsorption to the bacterium).
Through virions attacking the cell wall, lysozymes and muramidases negatively affect bacterial aggregation, adhesion, and subsequent biofilm formation. Muramidase in particular reduces adhesion in biofilms.
The SleB protein degrades the bacterial spore cortex. Thus, the presence of SleB and similar proteins in phages help to penetrate dormant bacterial populations within the biofilm environment.
Depolymerase proteins or the tail spike/ fiber proteins, are characteristic of the Podoviridae family viruses. They are capable of cleaving bacterial polysaccharides: capsular polysaccharides (CPS), exopolysaccharides (EPS), and/or lipopolysaccharide (LPS), which are the main components of biofilms. The presence of these proteins in phage SI01 enables it to lyse the biofilm of the bacterium Stappia indica SNL01 with high efficiency.
Surprisingly, despite its virulent nature, the phage SI01 genome lacks tRNAs. This is fascinating as this fact contradicts the previous assumption that these molecules (tRNAs) are necessary for virulence.

When the scientists inoculated phage SI01 with Stappia indica SNL01 bacteria on a soft agar plate, they observed 0.5- to 1-mm clear spots within 12 hours of inoculation, indicating bacterial lysis. Through this, they deduced that phage SI01 indeed promoted the growth of diatom Thalassiosira pseudonana through bacterial lysis as evidenced by the results.
It was noticed that at higher concentrations of the phage, the growth-inhibiting effect of Stappia indica SNL01 was suppressed when the phage was introduced to the Thalassiosira pseudonana–S. indica SNL01 interaction system (resembling a biofilm).
Taking a step further, the researchers analysed the chlorophyll intensity of the diatom which correlated with the algal abundance in the sea. From the data obtained, they concluded that Stappia indica SNL01 inhibited the growth of diatom Thalassiosira pseudonana at higher concentrations (106 to 107 cells mL-1) as shown in the figure below (refer to the purple and green lines in panel a wherein the chlorophyll intensity declines and panel b wherein the chlorophyll intensity increases).

To better understand the growth cycle of the virus SI01, a one-step growth experiment was conducted. This experiment helped to understand the duration of the different phases of the virus and helped to analyse the infection pattern of phage SI01 in Stappia indica SNL01. The results demonstrated that the virus had a longer latent period (120 min) when compared to other marine Podoviridae phages, followed by a consequent lengthy burst period of around 150 min. The lengthy burst period and size of the virus, that is the duration and amount of virions produced by infected bacterial cells, reflect on the high infectivity of the virus.

To summarise, phage SI01 is truly a blossoming debutant in the field of marine microbiology. Its isolation and the subsequent research conducted on it, have certainly contributed to a better understanding of the impact of phage infection on the alga-bacterium relationship in oceans, which was previously poorly understood. Its ability to easily (and amazingly) adapt to fluctuating seasonal and geographical changes due to its inherent stable pH and temperature, makes the virus an enticing mystery to explore in virology research.
Link to the original post: Shailesh Nair et al (2022). A Novel Phage Indirectly Regulates Diatom Growth by Infecting a Diatom-Associated Biofilm-Forming Bacterium. Applied and Environmental Microbiology, American Society for Microbiology Journal, 88(5). Doi: https://doi.org/10.1128/aem.02138-21
Featured image: Made with BioRender.com and icons created by ultimatearm and Nikita Golubev available on flaticon.