Breaking down the microbiology world one bite at a time
Vibrio cholerae hunts immune cells using biofilm
Bacteria are small yet complex organisms. We know how different bacteria infect us all the time and our immune system then comes to the rescue! Immune system consists of cells like macrophages which circulate throughout our body and upon encountering bacteria, they engulf and eat them. But bacteria being the smart organism that they are, have evolved several strategies to evade the immune system.
When in a group, bacteria can talk to each other and adhere to each other to form biofilm by producing an extracellular matrix. The matrix of biofilm can act as a cover for bacteria to hide and protect themselves from the immune cells and the antibiotics that we take. However, exactly how bacterial biofilm interacts with our immune cells was not known until now…
Can bacteria form biofilm on immune cells?
In a study, Vidakovic and team tried to understand the interaction between immune cells and Vibrio cholerae, a bacterium that is known to form biofilm and cause diarrhea in humans.
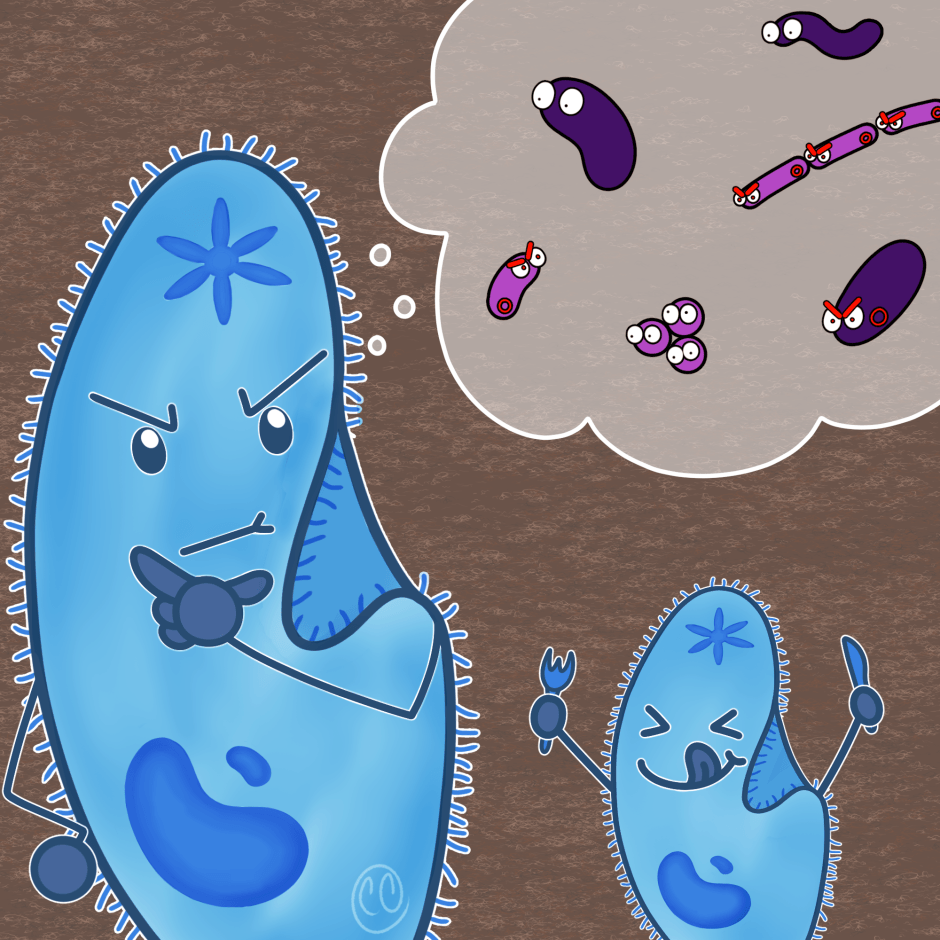
First, the authors checked whether Vibrio cholerae can form biofilm on our immune cells or not. Using microscopy, they found that V. cholerae attached to different immune cells. Over time, it formed a multilayer sheath- like biofilm on them. Interestingly, after the formation of biofilm, the bacterium killed the same immune cells and later dispersed in the surrounding to attach to others (Figure 1). This suggests that bacterial biofilm also hunts immune cells!

Authors then tried to find how Vibrio attaches to immune cells. Generally bacteria use molecules called adhesin to attach to the epithelium cells. But bacteria incapable of producing adhesin also form biofilm on immune cells using flagella and mannose-sensitive hemagglutinin (MSHA) or TC pili. These two appendages break off from the bacteria and become part of the biofilm matrix to enhance the stability of the biofilm.
How does bacteria use biofilm to kill immune cells?
Vibrio produces several toxins and one of them is hemolysin (HlyA). HlyA protein allows bacteria to attach and forms pores to kill the cells. But if these toxins can be produced by bacteria without forming biofilm, then why should bacteria form biofilm?
Here’s why.
The team found that biofilm formation on immune cells helps in the accumulation of HlyA toxin near them. Accumulated high doses of toxin within biofilm makes biofilm producing bacteria more effective at killing macrophages.
Later after killing immune cells, the level of c-di-GMP, a small molecule found in various signaling pathways, decreased. This caused the biofilm to disperse thus helping in the dispersal of bacterial cells.
How does Vibrio encounter macrophages?
Vibrio colonizes the intestinal surface, while immune cells reside inside. But then how does Vibrio cholerae come into contact with the immune cells? Does the bacterium break the intestinal epithelium barrier and then encounter the immune cells?
To address these questions, scientists used a setup shown in Figure 2. In this setup, they grew macrophages at the base, and intestinal epithelium cells from a human small intestinal organoid, on a permeable top surface. They infected the top epithelium layer with Vibrio cholerae. Using microscopy, they saw that Vibrio could break through the epithelium layer to reach the immune cells and kill them.

Image source: Vidakovic L et al (2023)
Overall, this study showed that Vibrio cholerae forms biofilm on the immune cells. For the first time, bacterial biofilm is shown as a predation strategy to kill immune cells, reducing their number in their surroundings (Figure 3). Thus, this study revealed the reversal role of bacteria being the hunter and immune cells hunted.

Link to the original post: Vidakovic L, Mikhaleva S, Jeckel H, Nisnevich V, Strenger K, Neuhaus K, Raveendran K, Ben-Moshe NB, Aznaourova M, Nosho K, Drescher A, Schmeck B, Schulte LN, Persat A, Avraham R, Drescher K. Cell, 2023 Jun 8. 10.1016/j.cell.2023.05.008
Featured image: Anthony D’Onofrio from Flickr