Breaking down the microbiology world one bite at a time
How to win an arms race: lessons from “pink berries”
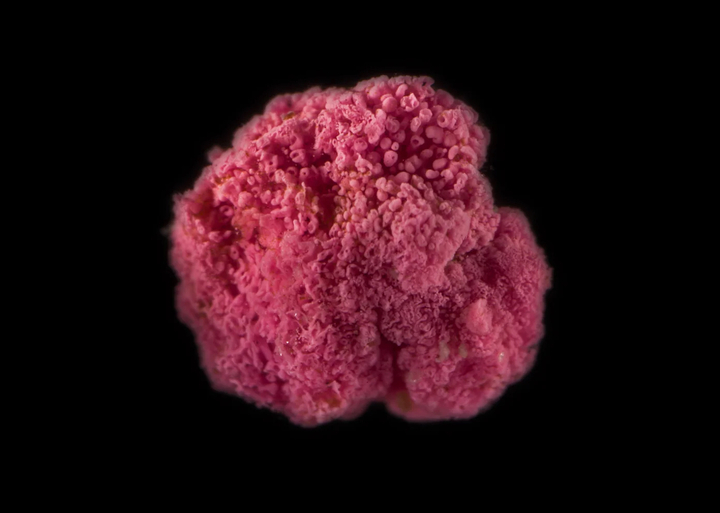
In tidal pools in the high salt marsh in Cape Cod, one might discover curious bright pink berries resting in the mud. These mysterious “pink berries” are not berries at all—instead, they are bacterial communities that hold insight into the evolution of multi-cellular life.

There is plenty of mystery surrounding these “berries”. For one, they are a dense population of just a few species—meaning that most organisms in the berries are closely related to each other. As we learned during the COVID-19 pandemic, this is a situation that viruses are well situated to exploit. Without social distancing and masks, how do the bacteria in pink berries (and by extension other forms of multicellular life) protect themselves from viral attack?
Pink berries are an excellent model for the evolution of multicellular life in general. Given this, a team of researchers set out to characterize one of the most abundant organisms comprising the pink berries: PB-PSB1, which is a phototrophic purple sulfur bacterium, meaning that it uses light and sulfur to harvest energy. What they found in PB-PSB1’s enormous genome challenges our perception of bacterial immune function. This bacterium appears to hone its anti-viral sensors using sophisticated mechanisms for rapid and targeted mutation.
Keeping up with the phages
To explain their discovery, we’ll need to understand something called a “diversity generating retroelement” (DGR). A retroelement is a piece of DNA that moves around in the genome of its host organism by being transcribed into RNA, then “reverse-transcribed” back into DNA and re-inserted in a new location in the genome. DGR are retroelements that are reverse-transcribed by an enzyme that specializes in being error prone—basically, it has evolved to be bad at its job. This means that when the DNA is reinserted into the genome, its sequence has been randomly changed. Each DGR targets a specific region of a gene. This region experiences frequent random mutations.

Why would a cell want to generate random mutations? In general, large amounts of random mutation aren’t good. We rely on proteins to do their job, and not to have random, function-changing mutations. For some types of sequences though, this random mutation can be a good thing.
Imagine a protein that acts as a sensor. It can bind to certain sequences of DNA and in doing so, detect the presence of these sequences. Now, imagine that the sequence the protein is looking for is rapidly evolving to evade detection. In that scenario, it might be extremely beneficial for the region of the sensor to have frequent mutations, so that some of its mutant forms will be likely to bind to the rapidly mutating target DNA that it hopes to recognize.
This is the case for the “arms race” between viruses and the cells they hope to infect. Viruses are constantly mutating in hopes of evading detection, while their hosts are trying to maintain the ability to detect them.
A new form of bacterial adaptive immunity?
In the genome of PB-PSB1, the authors of this new study noticed something striking: it contains nine DGRs targeting regions in fifteen genes. This is remarkable—only 0.04% of studied bacteria contain more than two complete DGRs. Intrigued, they took a closer look at the target genes of this multitude of DGRs to understand their function.
They discovered that most of the target genes of PB-PSB1’s DGRs likely function as antigen sensors, meaning that they detect unwelcome DNA from a viral invader so that the cell can respond to infection. Targeted mutation of such sensors is quite rare. This discovery indicates that some bacteria may have a more sophisticated and complex immune system than previously realized. In fact, if the authors of this study are correct, they have discovered a system that in some ways functions similarly to vertebrate adaptive immunity.
The bigger picture
This strategy may turn out to be widespread among bacteria living in multi-cellular consortia. By looking for related DGRs in published sequences, the researchers have identified potentially similar immune functions in a number of other bacteria. A large proportion (82%) of bacteria that contained DGRs related to PB-PSB1 have multi-cellular lifestyles.
It is evolutionarily favorable to bacteria with multicellular lifestyles to prioritize the survival of the most cells in the community over each individual cell, as seen in similar life forms, like animals and plants that experience similar evolutionary pressures. The abundance of DGRs in these bacteria indicates that this mode of immune function is particularly important for multicellular life.
Considering the challenge represented by living near very close genetic relatives, it is not surprising that sophisticated immune function is essential among these organisms. The discovery of such sophisticated immune mechanisms is nonetheless striking and indicates that mutation-based adaptive immunity evolved earlier, and is more widespread than previously thought.
Link to the original post: Doré, H., Eisenberg, A. R., Junkins, E. N., Leventhal, G. E., Ganesh, A., Cordero, O. X., Paul, B. G., Valentine, D. L., O’Malley, M. A., & Wilbanks, E. G. (2024). Targeted hypermutation of putative antigen sensors in multicellular bacteria. Proceedings of the National Academy of Sciences, 121(9), e2316469121. https://doi.org/10.1073/pnas.2316469121
Featured image: Image source: wilbankslab.org