
Breaking down the microbiology world one bite at a time
Gut Inflammation Disrupts Hormone Signals and the Brain
A few years ago, Dr. Annie Ciernia and Dr. Carolina Tropini met at a faculty mixer at the University of British Columbia. Their research areas, brain development and the gut microbiome respectively, were seemingly at odds. Yet, their conversation sparked a collaborative project that resulted in a new study published earlier this year which revealed a surprising connection between gut inflammation, brain development and hormone regulation.
When Ciernia and Tropini first set out to explore the effects of gut inflammation on brain development, they expected to find changes in behavior. They were inspired by the known overlap between immune dysfunction, gastrointestinal disorders, and neurodevelopmental conditions like autism. For that reason, they took DSS—a well-established chemical that disrupts the gut’s mucus lining and causes inflammation similar to inflammatory bowel disease (IBD)—and used it on young mice to disrupt their gut during development. Then, they tested how their learning, anxiety, social and repetitive behaviors changed due to the inflammation and tried to relate those findings to changes in the brain and gut microbiome.
But to their surprise, “Everything in the mouse’s behavior looked normal,” said Dr. Ciernia in our interview. “That’s good news for families dealing with children that have early-life inflammation, like IBD. Their behavioral development seemed to proceed as expected”, she assured.
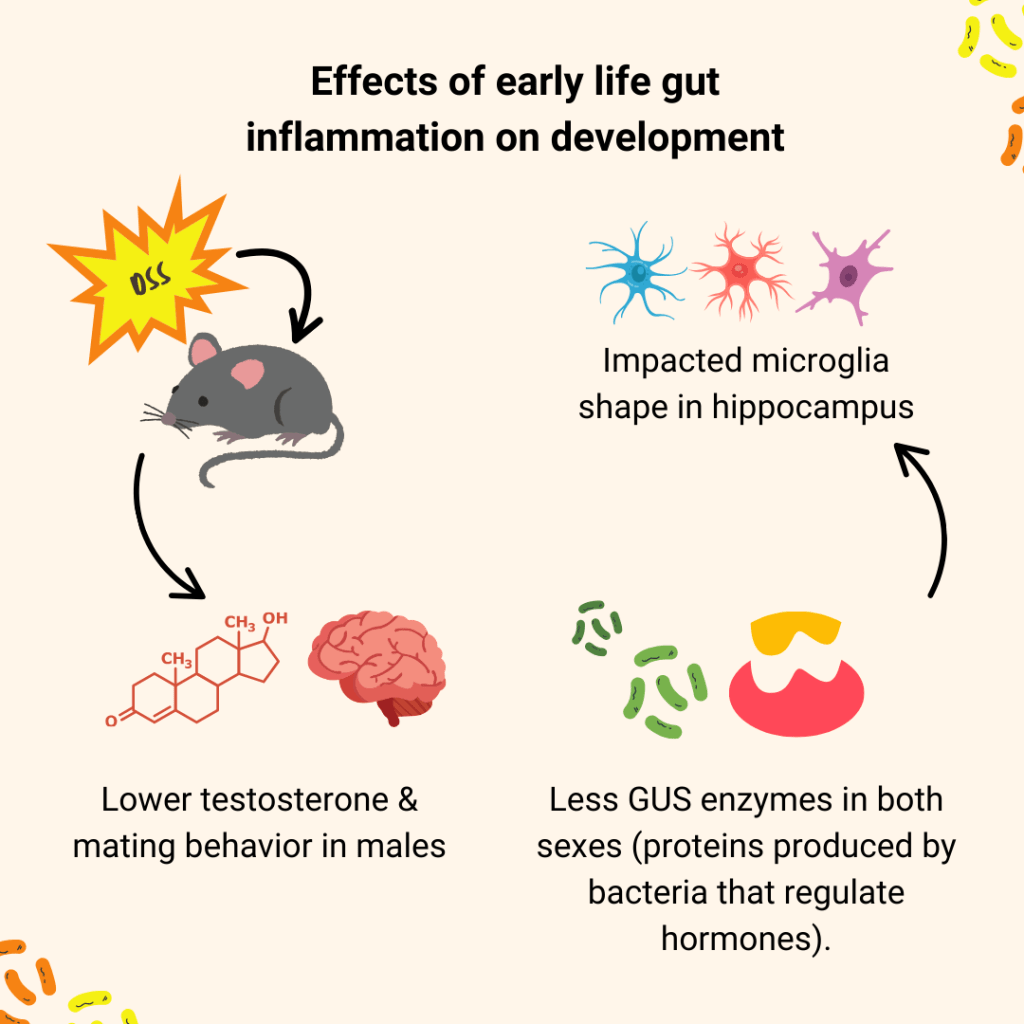
Later, as one of Dr. Ciernia’s graduate students looked more closely at their mice, they noticed something strange; in male mice exposed to early-life gut inflammation, the seminal vesicles (key sex organs) were unusually small and their testosterone levels were lower than controls. Something was happening, just not where they first expected to see it.
“We thought we were doing a neurodevelopment study,” Dr. Ciernia laughed. “But it turns out, we were doing a neuro-endocrine one.” The endocrine system is responsible for hormonal production in the body, including testosterone. This unexpected shift became the focus of the study. They did several experiments to track the animals’ hormone levels, gene expression, and microbiome composition. What they found was a clear disruption in mating behavior and the endocrine system; Specifically, male mice had reduced interest in the scent of female mice and reduced activity of a protein produced by bacteria in the gut known as: “GUS enzymes”. GUS enzymes normally help recycle sex hormones (like testosterone) back into the bloodstream.
The lack of GUS could explain the delayed development of reproductive tissues and lower testosterone levels observed in the males. Dr. Ciernia theorized that it might even help shed light on why boys with IBD are more likely to experience delayed puberty. “In IBD patients, puberty delays and infertility are common, but we don’t fully understand why,” she explains. “This may be one of the missing pieces. The microbiome helps regulate hormone availability and gut inflammation throws that balance off.”
Interestingly, female mice didn’t show the same reproductive hormone changes, even though both sexes experienced brain inflammation. That difference led the team to dig deeper into sex-specific effects. They found that microglia (immune cells in the brain) also responded differently by sex. “We think this neuroendocrine component has been overlooked,” Dr. Ciernia shared. “People tend to silo hormones, brain, and immune function, but they’re all connected”. She remarked that this study is a reminder for neuroscientists to look at the whole body.
Despite changes in hormone activity, the researchers didn’t see major behavioral shifts in older mice either. That may actually be reassuring. “It suggests a certain level of resilience,” Ciernia says. “The mice seem to have recovered, at least partially”. She emphasized that early gut inflammation could still affect growth and reproductive health in subtle ways. “It’s important to monitor milestones closely, especially in kids with chronic inflammation. Hormones regulate so many systems, and disruptions during critical periods might have long-term consequences.” A general overview of their proposed mechanism can be found in Figure 1.
So what’s next? The team has already begun testing their hypothesis on a germ-free mouse model, i.e. mice born without any microbes at all. Their goal is to transplant fecal matter from humans with IBD into the germ-free mice, and see if the microbiota alone is enough to cause the same hormonal disruptions in the mice. If true, this would prove that there is a causal link between the IBD microbiome and shifts in hormonal production, as well as brain inflammation.
Perhaps most intriguingly, the study hints at new therapeutic directions. Could probiotics or microbiome-based treatments help restore hormone balance in IBD patients? Dr. Ciernia agrees: “If we can figure out how gut microbes influence endocrine function, we might find a way to support kids going through early life inflammation.”
As we continue to learn more about the complex field of neurodevelopment and the microbiome, I asked Dr. Ciernia for her advice to future scientists and scholars interested in this research. “I suggest that they be willing to follow the weird and unexpected findings,” she says. “It might just lead you into a whole new field.”
Link to the original post: Sullivan, O., Sie, C., Ng, K. M., Cotton, S., Rosete, C., Hamden, J. E., … Tropini, C. & Ciernia, A. V. (2025). Early-life gut inflammation drives sex-dependent shifts in the microbiome-endocrine-brain axis. Brain, behavior, and immunity, 125, 117-139. https://doi.org/10.1016/j.bbi.2024.12.003
Featured image: Created by the author using Canva Pro.















