Breaking down the microbiology world one bite at a time
The healing bacterium
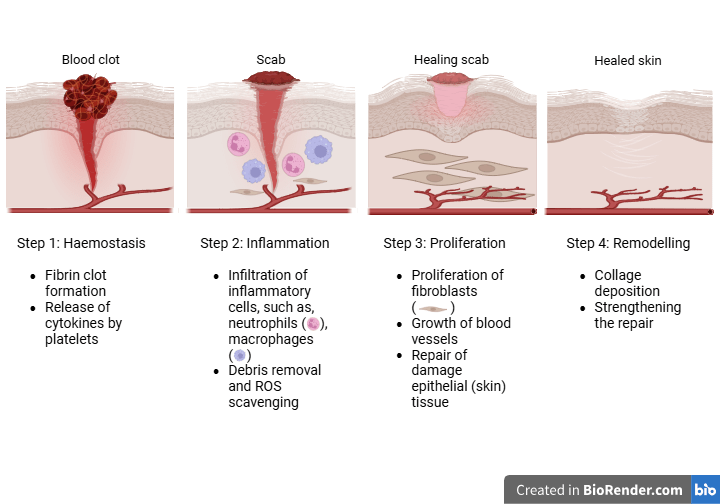
Wounds, breaks or openings in the living tissues, are common injuries for human beings. They can be caused by falls, accidents, or surgeries. These open cuts can serve as a path for various bacteria and germs to enter and cause infections. To prevent these infections, the body has a fully developed wound healing process that involves 4 steps, namely haemostasis, inflammation, proliferation, and remodelling, as explained in the image below. The process takes a few weeks.
During the haemostasis phase, inflammatory cells, growth factors, and fibrin stop the flow of blood and lead to clot formation. Additional immune cells, such as neutrophils and macrophages, are enlisted during the inflammatory phase. The wound then re-epithelializes during the proliferative phase, during which granulation tissue and blood vessels develop and grow. Scar tissue is created when the wound contracts towards the end of the remodelling phase.
Some wounds, such as in the case of diabetes, spinal cord injuries, or a weak immune system, do not heal within the natural timeframe and are known as chronic wounds. Chronic wounds have become a more significant clinical issue in recent decades as a result of their increased occurrence and related deaths. As discussed in a previous study by White and Grice the wound microbiome, which is an ecosystem of many bacterial species that colonize the wound, is a ubiquitous part of the wound environment and impacts wound healing. It is important to understand the interactions and dynamics between these microbes to comprehend how they affect healing.
While the most abundant bacterial species found in the wound microbiome, Streptococcus aureus and Psuedomonas aeruginosa are highly infectious, other species, such as Corynebacterium striatum and Propionibacterium spp. are human skin commensals that can promote skin repair. Another bacterium found frequently in chronic wounds is Alcaligenes faecalis, a non-pathogenic environmental bacterium.
Following this information, White and her team worked on a diabetic mouse model with impaired wound healing. The wounds on a diabetic mouse were treated with the clinical isolate of A. faecalis and studied for 3 weeks. It was found that the wounds treated with the bacterium showed signs of accelerated wound closure from as early as day 3 as compared to the untreated wounds. Further, the wounds treated with the bacterium did not show any signs of infection, had well-maintained margins, and had a healthy wound bed. While A. faecalis proliferated in the early days of wound healing, the inflammatory responses that followed completely cleared A. faecalis by the end of the later days.
This finding led the researchers on a quest to unravel the mechanism behind the wound closure process driven by A. faecalis. It was observed that the effects of the bacterium were strongest during the early re-epithelialization stage of wound healing, which is the process of restoring the epithelial cells (cells covering the skin) on a wounded site, see the image below. The cellular and molecular mechanisms necessary for successful wound closure include initiation, migration, maintenance, cellular differentiation, and completion of epithelialization. Growth factors, cytokines, matrix metalloproteinases, cellular receptors, and extracellular matrix constituents are among the many modulators at play.
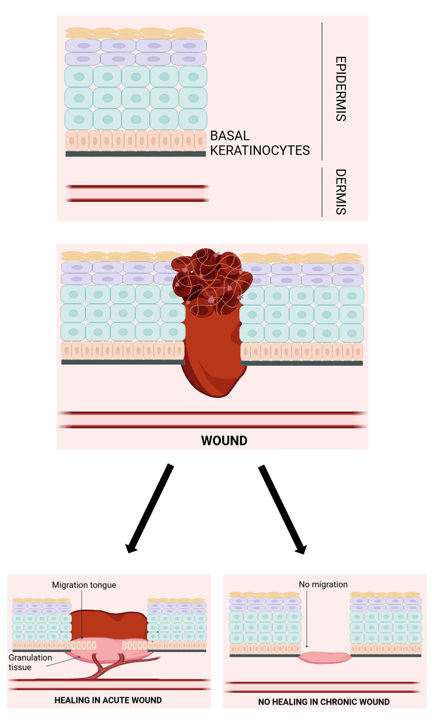
The authors observed that A. faecalis – treated wounds showed significant increases in both keratinocyte (epidermal skin cells) migration and proliferation (processes that are impaired in diabetic patients) as compared to the untreated wounds. Upon further testing, similar results were also obtained on human skin.
In addition to this, experiments revealed that A. faecalis immensely upregulated several genes related to leukocyte recruitment and activation of T cells. The most upregulated were the immune cells CD4+ T cells and neutrophils. On the other hand, the most downregulated genes were those associated with matrix metalloproteins (MMPs). Keratinocytes near the leading edge of the wound expressed Mmp10, an MMP that breaks down the extracellular matrix (ECM) elements. These ECM components, i.e., proteins and other molecules surrounding and supporting the cells, serve as a substrate for keratinocyte migration during wound healing. However, diabetes and hyperglycemia stimulate increased MMP expression, which is detrimental to wound healing.
The findings of this study collectively imply that A. faecalis facilitates diabetic wound healing by promoting re-epithelialization through local inhibition of Mmp10 overexpression. Based on further assays, it was confirmed that A. faecalis lowers Mmp10 at the wound site to accomplish its pro-healing effects.
The novelty of this study lies in the fact that it highlights the positive aspects of chronic wound microbiomes. The work uncovered the mechanism by which A. faecalis promotes early wound healing, thereby providing a groundwork for developing microbiome-based treatment methods.
Link to the original post: Original article: E.K. White, A. Uberoi, J.T.C. Pan, J.T. Ort, A.E. Campbell, S.M. Murga-Garrido, J.C. Harris, P. Bhanap, M. Wei, N.Y. Robles, S.E. Gardner, E.A. Grice. Alcaligenes faecalis corrects aberrant matrix metalloproteinase expression to promote reepithelialization of diabetic wounds. Science Advances, 10, eadj2020, June 2024. DOI: 10.1126/sciadv.adj2020.
Featured image: Created by the author using Canva
Additional sources:
E.K. White, E.A. Grice. The wound microbiome. Cold Spring Harbor Perspectives in Biology, 15(6), a041218, June 2023. DOI: 10.1101/cshperspect.a041218.
V. Falanga, R.R. Isseroff, A.M. Soulika, M. Romanelli, D. Margolis, S. Kapp, M. Granick, K. Harding. Chronic wounds primers. Nature Reviews Disease Primers, 8, 50, July 2022. DOI: 10.1038/s41572-022-00377-3.